Abstract
Introduction
5-Fluoro-2′-deoxycytidine (FdCyd; NSC48006), a fluoropyrimidine nucleoside inhibitor of DNA methylation, is degraded by cytidine deaminase (CD). Pharmacokinetic evaluation was carried out in cynomolgus monkeys in support of an ongoing phase I study of the PO combination of FdCyd and the CD inhibitor tetrahydrouridine (THU; NSC112907).
Methods
Animals were dosed intravenously (IV) or per os (PO). Plasma samples were analyzed by LC–MS/MS for FdCyd, metabolites, and THU. Clinical chemistry and hematology were performed at various times after dosing. A pilot pharmacokinetic study was performed in humans to assess FdCyd bioavailability.
Results
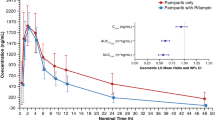
After IV FdCyd and THU administration, FdCyd C max and AUC increased with dose. FdCyd half-life ranged between 22 and 56 min, and clearance was approximately 15 mL/min/kg. FdCyd PO bioavailability after THU ranged between 9 and 25 % and increased with increasing THU dose. PO bioavailability of THU was less than 5 %, but did result in plasma concentrations associated with inhibition of its target CD. Human pilot studies showed comparable bioavailability for FdCyd (10 %) and THU (4.1 %).
Conclusion
Administration of THU with FdCyd increased the exposure to FdCyd and improved PO FdCyd bioavailability from <1 to 24 %. Concentrations of THU and FdCyd achieved after PO administration are associated with CD inhibition and hypomethylation, respectively. The schedule currently studied in phase I studies of PO FdCyd and THU is daily times three at the beginning of the first and second weeks of a 28-day cycle.



Similar content being viewed by others
References
Mekras JA, Boothman DA, Perez LM, Greer S (1984) Use of 5-fluorodeoxycytidine and tetrahydrouridine to exploit high levels of deoxycytidylate deaminase in tumors to achieve DNA- and target-directed therapies. Cancer Res 44(6):2551–2560
Boothman DA, Briggle TV, Greer S (1987) Tumor-selective metabolism of 5-fluoro-2’-deoxycytidine coadministered with tetrahydrouridine compared to 5-fluorouracil in mice bearing Lewis lung carcinoma. Cancer Res 47(9):2354–2362
Boothman DA, Briggle TV, Greer S (1987) Protective, tumor-selective dual pathway activation of 5-fluoro-2’-deoxycytidine provided by tetrahydrouridine in mice bearing mammary adenocarcinoma-755. Cancer Res 47(9):2344–2353
Kaysen J, Spriggs D, Kufe D (1986) Incorporation of 5-fluorodeoxycytidine and metabolites into nucleic acids of human MCF-7 breast carcinoma cells. Cancer Res 46(9):4534–4538
Smith SS, Kaplan BE, Sowers LC, Newman EM (1992) Mechanism of human methyl-directed DNA methyltransferase and the fidelity of cytosine methylation. Proc Natl Acad Sci USA 89(10):4744–4748
Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, Jones PA (2005) Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2’-deoxycytidine. Mol Cancer Ther 4(10):1515–1520. doi:10.1158/1535-7163.MCT-05-0172
Beumer JH, Eiseman JL, Parise RA, Joseph E, Holleran JL, Covey JM, Egorin MJ (2006) Pharmacokinetics, metabolism, and oral bioavailability of the DNA methyltransferase inhibitor 5-fluoro-2’-deoxycytidine in mice. Clin Cancer Res Off J Am Assoc Cancer Res 12(24):7483–7491. doi:10.1158/1078-0432.CCR-06-1250
Kinders RJ, Wang L, Kummar S, Khin S, Balasubramanian P, Zhu W, Parchment RE, Newman E, Tomaszewski JE, Doroshow JH (2011) Abstract A106: Investigation of 5-fluorodeoxycytidine with tetrahydrouracil as a demethylation regimen in solid tumors. Mol Cancer Ther 10(Suppl 1):A106–A106. doi:10.1158/1535-7163.targ-11-a106
Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R 3rd, Shen L, Nimer SD, Leavitt R, Raza A, Saba H (2006) Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 106(8):1794–1803. doi:10.1002/cncr.21792
Quintas-Cardama A, Santos FP, Garcia-Manero G (2010) Therapy with azanucleosides for myelodysplastic syndromes. Nature Rev Clin Oncol 7(8):433–444. doi:10.1038/nrclinonc.2010.87
Newman EM, Morgan RJ, Kummar S, Beumer JH, Blanchard MS, Ruel C, El-Khoueiry AB, Carroll MI, Hou JM, Li C, Lenz HJ, Eiseman JL, Doroshow JH (2015) A phase I, pharmacokinetic, and pharmacodynamic evaluation of the DNA methyltransferase inhibitor 5-fluoro-2’-deoxycytidine, administered with tetrahydrouridine. Cancer Chemother Pharmacol 75(3):537–546. doi:10.1007/s00280-014-2674-7
Beumer JH, Parise RA, Newman EM, Doroshow JH, Synold TW, Lenz HJ, Egorin MJ (2008) Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2’-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU). Cancer Chemother Pharmacol 62(2):363–368. doi:10.1007/s00280-007-0603-8
Beumer JH, Eiseman JL, Parise RA, Florian JA Jr, Joseph E, D’Argenio DZ, Parker RS, Kay B, Covey JM, Egorin MJ (2008) Plasma pharmacokinetics and oral bioavailability of 3,4,5,6-tetrahydrouridine, a cytidine deaminase inhibitor, in mice. Cancer Chemother Pharmacol 62(3):457–464. doi:10.1007/s00280-007-0625-2
Parise RA, Egorin MJ, Eiseman JL, Joseph E, Covey JM, Beumer JH (2007) Quantitative determination of the cytidine deaminase inhibitor tetrahydrouridine (THU) in mouse plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 21(13):1991–1997. doi:10.1002/rcm.3054
Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE (1966) Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Reports Part 1 50 (4):219–244
De Verdier CH, Potter VR (1960) Alternative pathways of thymine and uracil metabolism in the liver and hepatoma. J Natl Cancer Inst 24:13–29
Garcia-Manero G, Stoltz ML, Ward MR, Kantarjian H, Sharma S (2008) A pilot pharmacokinetic study of oral azacitidine. Leukemia 22(9):1680–1684. doi:10.1038/leu.2008.145
Acknowledgments
Funding for these studies was provided by NCI contracts N01-CM-42202 (IIT Research Institute), N01-CM-52202, N01CM-2011-0015, UM1CA186690 (University of Pittsburgh), and UM1CA186717 (City of Hope). This project used the UPCI Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30CA047904. The authors would like to thank the Merrill Egorin Writing Group at UPCI for careful review of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Holleran, J.L., Beumer, J.H., McCormick, D.L. et al. Oral and intravenous pharmacokinetics of 5-fluoro-2′-deoxycytidine and THU in cynomolgus monkeys and humans. Cancer Chemother Pharmacol 76, 803–811 (2015). https://doi.org/10.1007/s00280-015-2857-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2857-x