Abstract
Purpose
To assess the link between tumor growth inhibition (TGI) and overall survival (OS) based on historical renal cell carcinoma (RCC) data. To illustrate how simulations can help to identify TGI thresholds based on target OS benefit [i.e., hazard ratio (HR) compared with standard of care] to support new drug development in RCC.
Methods
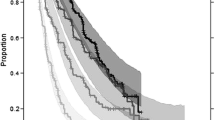
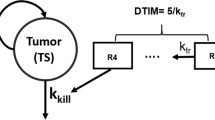
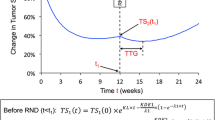
Tumor size (TS) data were modeled from 2552 patients with first-line or refractory RCC who received temsirolimus, interferon, sunitinib, sorafenib or axitinib in 10 Phase II or Phase III studies. Three model-based TGI metrics estimates [early tumor shrinkage (ETS) at week 8, 10 or 12, time to tumor growth (TTG) and growth rate] as well as baseline prognostic factors were tested in multivariate lognormal models of OS. Model performance was evaluated by posterior predictive check of the OS distributions and hazard ratio across treatments.
Results
TTG was the best TGI metric to predict OS. However, week 8 ETS had a satisfactory performance and was employed in order to maximize clinical utilization. The week 8 ETS to OS model was then used to simulate clinically relevant ETS thresholds for future Phase II studies with investigational treatments.
Conclusions
The published OS model and resultant simulations can be leveraged to support Phase II design and predict expected OS and HR (based on early observed TGI data obtained in Phase II or Phase III studies), thereby informing important mRCC development decisions, e.g., Go/No Go and dose regimen selection.



Similar content being viewed by others
References
Bruno R, Mercier F, Claret L (2014) Evaluation of tumor-size response metrics to predict survival in oncology clinical trials. Clin Pharmacol Ther 95:386–393
Bruno R, Lu JF, Sun YN, Claret L (2011) A modeling and simulation framework to support early clinical drug development decisions in oncology. J Clin Pharmacol 51:6–8
Stein WD, Wilkerson J, Kim ST et al (2012) Analyzing the pivotal trial that compared sunitinib and IFN-α in renal cell carcinoma, using a method that assesses tumor regression and growth. Clin Cancer Res 18:2374–2381
Stein A, Wang W, Carter AA et al (2012) Dynamic tumor modeling of the dose–response relationship for everolimus in metastatic renal cell carcinoma using data from the phase 3 RECORD-1 trial. BMC Cancer 12:311
Stein A, Bellmunt J, Escudier B et al (2013) Survival prediction in everolimus-treated patients with metastatic renal cell carcinoma incorporating tumor burden response in the RECORD-1 trial. Eur Urol 64:994–1002
Maitland ML, Wu K, Sharma MR et al (2013) Estimation of renal cell carcinoma treatment effects from disease progression modeling. Clin Pharmacol Ther 93:345–351
Hudes G, Carducci M, Tomczak P et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2278
Motzer RJ, Rini BI, Bukowski RM et al (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295:2516–2524
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Motzer RJ, Hutson TE, Tomczak P et al (2009) Overall survival and updated results for sunitinib compared with IFN-alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27:3584–3590
Motzer RJ, Hutson TE, Olsen MR et al (2012) Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol 30:1371–1377
Study of SU011248 in patients with advanced kidney cancer (A6181072). http://clinicaltrials.gov/ct2/show/study/NCT00254540
A continuation study using sunitinib malate for patients leaving treatment on a previous sunitinib study (6181110). http://clinicaltrials.gov/ct2/show/study/NCT00428220
Rixe O, Bukowski RM, Michaelson MD et al (2007) Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol 8:975–984
Rini BI, Wilding G, Hudes G et al (2009) Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol 27:4462–4468
Rini BI, Escudier B, Tomczak P et al (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomized phase 3 trial. Lancet 378:1931–1939
Motzer RJ, Escudier B, Tomczak P et al (2013) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14:552–562
Tomita Y, Uemura H, Fujimoto H et al (2011) Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell carcinoma. Eur J Cancer 47:2592–2602
Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17:2530–2540
Bamias A, Tzannis K, Beuselinck B et al (2013) Development and validation of a prognostic model in patients with metastatic renal cell carcinoma treated with sunitinib: a European collaboration. Br J Cancer 109:332–341
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Stein WD, Gulley JL, Schlom J et al (2010) Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res 17:907–917
Claret L, Gupta M, Han K et al (2013) Evaluation of tumor size response metrics to predict overall survival in western and Chinese patients with first line metastatic colorectal cancer. J Clin Oncol 31:2110–2114
Wang Y, Sung C, Dartois C et al (2009) Elucidation of relationship between tumor size and survival in non-small cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther 86:167–174
Phoenix® NLME™ version 1.2 (Certara L.P. (Pharsight), St. Louis, MO)
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
An MW, Mandrekar SJ, Sargent DJ. Application of tumor measurement-based metrics in the real world (2013) J Clin Oncol 31:4374; reply by Claret L, Bruno R. J Clin Oncol 31:4374–4375
NCCN guidelines, Kidney Cancer (2014) Version 3.2015. http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
Venkatakrishnan K, Friberg LE, Ouellet D et al (2015) Optimizing oncology therapeutics through quantitative clinical pharmacology: challenges and opportunities. Clin Pharmacol Ther 97:37–54
Sharma MR, Gray E, Goldberg RM et al (2015) Resampling the N9741 trial to compare tumor dynamic versus conventional end points in randomized phase II trials. J Clin Oncol 33:36–41
Venook AP, Tabernero J (2015) Progression-free survival: helpful biomarker or clinically meaningless end point? J Clin Oncol 33:4–6
Acknowledgments
The authors thank one of the reviewers for helpful comments motivating additional analyses that improved the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research support
Pfizer Pharmacometrics.
Financial interest disclosures
LC, FM and RB were employees of Pharsight Consulting Services at the time this research was conducted and were paid contractors to Pfizer in connection with the study and the development of this manuscript. BH and PM are full-time employees of Pfizer and may hold stocks or stock options.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Claret, L., Mercier, F., Houk, B.E. et al. Modeling and simulations relating overall survival to tumor growth inhibition in renal cell carcinoma patients. Cancer Chemother Pharmacol 76, 567–573 (2015). https://doi.org/10.1007/s00280-015-2820-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2820-x