Abstract
Purpose
The aim of this study was to describe the nonlinear pharmacokinetics of total and unbound plasma cisplatin under different administered time in patients with non-small-cell lung carcinoma.
Methods
Patients receiving chemotherapy with cisplatin were included in this analysis. Patients were divided into two groups depending on the administrated time of cisplatin: 6:00 (Group A) and 18:00 (Group B). The population pharmacokinetics of cisplatin was calculated using nonlinear mixed-effects modeling (NONMEM) method, and the possible influence of covariates on the population pharmacokinetics of cisplatin was also explored.
Results
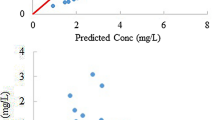
The pharmacokinetics of total and unbound cisplatin could be described well by a linear two-compartment model. The mean population estimates for total and unbound drug were, respectively, 0.463 (17.0 %) and 25.4 (14.0 %) l h−1 for clearance (CL), 24.2 (19.9 %) and 20.5 (27.1 %) l for central distribution volume (V 1), 10.2 (18.2 %) and 9.82 (28.1) l h−1 for intercompartmental clearance (Q) and 32.0 (24.1 %) and 6.77 (25.4 %) l for peripheral compartment volume (V 2). The CL for total and unbound cisplatin was dependent on body surface area (BSA). When cisplatin was administered at 18:00, the CL was 1.38- and 1.22-fold higher than those administered at 6:00 for total and unbound cisplatin, respectively (P < 0.05). The mean parameter estimates from a nonparametric bootstrap procedure were comparable and within 5 % of the estimates from NONMEM.
Conclusions
The results showed that circadian could influence the metabolism of cisplatin and suggested the conventional dose adjustment of cisplatin based on BSA.








Similar content being viewed by others
References
Canaple L, Kakizawa T, Laudet V (2003) The days and nights of cancer cells. Cancer Res 63:7545–7552
Levi F (2001) Circadian chronotherapy for human cancers. Lancet Oncol 2:307–315
Hrushesky WJ (1985) Circadian timing of cancer chemotherapy. Science 228:73–75
Levi F (2006) Chronotherapeutics: the relevance of timing in cancer therapy. Cancer Causes Control 17:611–621
Levi F, Benavides M, Chevelle C, Le Saunier F, Bailleul F, Misset JL, Regensberg C, Vannetzel JM, Reinberg A, Mathe G (1990) Chemotherapy of advanced ovarian cancer with 4′-O-tetrahydropyranyl doxorubicin and cisplatin: a randomized phase II trial with an evaluation of circadian timing and dose-intensity. J Clin Oncol 8:705–714
Satoh T, Omuro Y, Sasaki Y, Hamamoto Y, Boku N, Tamura T, Ohtsu A (2012) Pharmacokinetic analysis of capecitabine and cisplatin in combination with trastuzumab in Japanese patients with advanced HER2-positive gastric cancer. Cancer Chemother Pharmacol 69:949–955
Sebestyen J, Garg U, Lewing KB, Warady BA, Abdel-Rahman S, Blowey DL (2010) Cisplatin pharmacokinetics in a child receiving peritoneal dialysis. Pediatr Nephrol 25:1185–1189
Verschraegen CF, Skubitz K, Daud A, Kudelka AP, Rabinowitz I, Allievi C, Eisenfeld A, Singer JW, Oldham FB (2009) A phase I and pharmacokinetic study of paclitaxel poliglumex and cisplatin in patients with advanced solid tumors. Cancer Chemother Pharmacol 63:903–910
Specenier PM, Ciuleanu T, Latz JE, Musib LC, Darstein CL, Vermorken JB (2009) Pharmacokinetic evaluation of platinum derived from cisplatin administered alone and with pemetrexed in head and neck cancer patients. Cancer Chemother Pharmacol 64:233–241
Urien S, Brain E, Bugat R, Pivot X, Lochon I, Van ML, Vauzelle F, Lokiec F (2005) Pharmacokinetics of platinum after oral or intravenous cisplatin: a phase 1 study in 32 adult patients. Cancer Chemother Pharmacol 55:55–60
Bonetti A, Franceschi T, Apostoli P, Messori A, Sperotto L, Cetto GL, Molino A, Leone R (1995) Cisplatin pharmacokinetics using a five-day schedule during repeated courses of chemotherapy in germ cell tumors. Ther Drug Monit 17:25–32
Ramon-Lopez A, Escudero-Ortiz V, Carbonell V, Perez-Ruixo JJ, Valenzuela B (2012) Population pharmacokinetics applied to optimising cisplatin doses in cancer patients. Farm Hosp 36:392–402
Urien S, Lokiec F (2004) Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br J Clin Pharmacol 57:756–763
de Jongh FE, Gallo JM, Shen M, Verweij J, Sparreboom A (2004) Population pharmacokinetics of cisplatin in adult cancer patients. Cancer Chemother Pharmacol 54:105–112
Ishibashi T, Yano Y, Oguma T (2003) Population pharmacokinetics of platinum after nedaplatin administration and model validation in adult patients. Br J Clin Pharmacol 56:205–213
Hanada K, Nishijima K, Ogata H, Atagi S, Kawahara M (2001) Population pharmacokinetic analysis of cisplatin and its metabolites in cancer patients: possible misinterpretation of covariates for pharmacokinetic parameters calculated from the concentrations of unchanged cisplatin, ultrafiltered platinum and total platinum. Jpn J Clin Oncol 31:179–184
Nagai N, Ogata H, Wada Y, Tsujino D, Someya K, Ohno T, Masuhara K, Tanaka Y, Takahashi H, Nagai H, Kato K, Koshiba Y, Igarashi T, Yokoyama A, Kinameri K, Kato T, Kurita Y (1998) Population pharmacokinetics and pharmacodynamics of cisplatin in patients with cancer: analysis with the NONMEM program. J Clin Pharmacol 38:1025–1034
Kurihara N, Kubota T, Hoshiya Y, Otani Y, Ando N, Kumai K, Kitajima M (1996) Pharmacokinetics of cis-diamminedichloroplatinum (II) given as low-dose and high-dose infusions. J Surg Oncol 62:135–138
Zhang M (1992) Clinical pharmacokinetics of cis-diamminedichloroplatinum (DDP). Zhonghua Zhong Liu Za Zhi 14:67–69
Ma J, Stoter G, Verweij J, Schellens JH (1996) Comparison of ethanol plasma-protein precipitation with plasma ultrafiltration and trichloroacetic acid protein precipitation for the measurement of unbound platinum concentrations. Cancer Chemother Pharmacol 38:391–394
LeRoy AF, Wehling ML, Sponseller HL, Friauf WS, Solomon RE, Dedrick RL, Litterst CL, Gram TE, Guarino AM, Becker DA (1977) Analysis of platinum in biological materials by flameless atomic absorption spectrophotometry. Biochem Med 18:184–191
Parke J, Holford NH, Charles BG (1999) A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Prog Biomed 59:19–29
Bonate PL (1999) The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm Res 16:709–717
Ikeda K, Terashima M, Kawamura H, Takiyama I, Koeda K, Takagane A, Sato N, Ishida K, Iwaya T, Maesawa C, Yoshinari H, Saito K (1998) Pharmacokinetics of cisplatin in combined cisplatin and 5-fluorouracil therapy: a comparative study of three different schedules of cisplatin administration. Jpn J Clin Oncol 28:168–175
Korst AE, van der Sterre ML, Gall HE, Fichtinger-Schepman AM, Vermorken JB, van der Vijgh WJ (1998) Influence of amifostine on the pharmacokinetics of cisplatin in cancer patients. Clin Cancer Res 4:331–336
Vermorken JB, van der Vijgh WJ, Klein I, Gall HE, van Groeningen CJ, Hart GA, Pinedo HM (1986) Pharmacokinetics of free and total platinum species after rapid and prolonged infusions of cisplatin. Clin Pharmacol Ther 39:136–144
Reece PA, Stafford I, Abbott RL, Anderson C, Denham J, Freeman S, Morris RG, Gill PG, Olweny CL (1989) Two- versus 24-hour infusion of cisplatin: pharmacokinetic considerations. J Clin Oncol 7:270–275
Belliveau J, Posner M, Ferrari L, Crabtree G, Cummings F, Wiemann M, O’Leary G Jr, Griffin H, Phaneuf M, O’Rourke A (1986) Cisplatin administered as a continuous 5-day infusion: plasma platinum levels and urine platinum excretion. Cancer Treat Rep 70:1215
Sprowl JA, van Doorn L, Hu S, van Gerven L, de Bruijn P, Li L, Gibson AA, Mathijssen RH, Sparreboom A (2013) Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin Pharmacol Ther. doi:10.1038/clpt.2013.145
Urakami Y, Okuda M, Saito H, Inui K (2000) Hormonal regulation of organic cation transporter OCT2 expression in rat kidney. FEBS Lett 473:173–176
Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A (2007) The association of time of day and serum testosterone concentration in a large screening population. BJU Int 100:509–513
Pabla N, Murphy RF, Liu K, Dong Z (2009) The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol 296:F505–F511
Landon CD, Benjamin SE, Ashcraft KA, Dewhirst MW (2013) A role for the copper transporter Ctr1 in the synergistic interaction between hyperthermia and cisplatin treatment. Int J Hyperthermia
Boughattas NA, Hecquet B, Fournier C, Bruguerolle B, Trabelsi H, Bouzouita K, Omrane B, Levi F (1994) Comparative pharmacokinetics of oxaliplatin (L-OHP) and carboplatin (CBDCA) in mice with reference to circadian dosing time. Biopharm Drug Dispos 15:761–773
Li XM, Metzger G, Filipski E, Boughattas N, Lemaigre G, Hecquet B, Filipski J, Levi F (1997) Pharmacologic modulation of reduced glutathione circadian rhythms with buthionine sulfoximine: relationship with cisplatin toxicity in mice. Toxicol Appl Pharmacol 143:281–290
Igarashi T, Izumi H, Uchiumi T, Nishio K, Arao T, Tanabe M, Uramoto H, Sugio K, Yasumoto K, Sasaguri Y, Wang KY, Otsuji Y, Kohno K (2007) Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene 26:4749–4760
Hrushesky WJ, Bjarnason GA (1993) Circadian cancer therapy. J Clin Oncol 11:1403–1417
Nagai N, Kinoshita M, Ogata H, Tsujino D, Wada Y, Someya K, Ohno T, Masuhara K, Tanaka Y, Kato K, Nagai H, Yokoyama A, Kurita Y (1996) Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol 39:131–137
Yamamoto N, Tamura T, Maeda M, Ando M, Shinkai T, Eguchi K, Ohe Y, Oshita F, Shiraishi J, Katsumata N et al (1995) The influence of ageing on cisplatin pharmacokinetics in lung cancer patients with normal organ function. Cancer Chemother Pharmacol 36:102–106
Acknowledgments
This work was supported by the nursing staff of the Oncology ward for their assistance. The authors appreciate the support of Ph.D Nan Hu.
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, R., Li, J., Hu, Ww. et al. Circadian variability of pharmacokinetics of cisplatin in patients with non-small-cell lung carcinoma: analysis with the NONMEM program. Cancer Chemother Pharmacol 72, 1111–1123 (2013). https://doi.org/10.1007/s00280-013-2288-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2288-5