Abstract
Purpose
Panobinostat, a pan-deacetylase inhibitor, increases acetylation of proteins associated with growth and survival of malignant cells. This phase 2 study evaluated the efficacy of intravenous (IV) panobinostat in patients with castration-resistant prostate cancer (CRPC) who had previously received chemotherapy. The primary end point was 24-week progression-free survival. Secondary end points included safety, tolerability, and the proportion of patients with a prostate-specific antigen (PSA) decline.
Methods
IV panobinostat (20 mg/m2) was administered to patients on days 1 and 8 of a 21-day cycle. Tumor response was assessed by imaging every 12 weeks (4 cycles) according to modified response evaluation criteria in solid tumors (Scher et al. in Clin Cancer Res 11:5223–5232, 23), and PSA response was defined as a 50 % decrease from baseline maintained for ≥4 weeks. Safety monitoring was routinely performed and included electrocardiogram monitoring.
Results
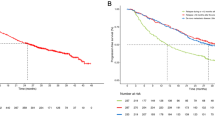
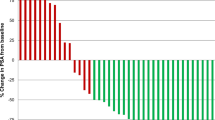
Of 35 enrolled patients, four (11.4 %) were alive without progression of disease at 24 weeks. PSA was evaluated in 34 (97.1 %) patients: five (14.3 %) patients demonstrated a decrease in PSA but none ≥50 %; one patient (2.9 %) had carcinoembryonic antigen as a marker of his prostate cancer, which declined by 43 %. Toxicities regardless of relationship to panobinostat included fatigue (62.9 %), thrombocytopenia (45.7 %), nausea (51.4 %), and decreased appetite (37.1 %).
Conclusions
Despite promising preclinical data and scientific rationale, treatment with IV panobinostat did not show a sufficient level of clinical activity to pursue further investigation as a single agent in CRPC.


Similar content being viewed by others
References
Albany C, Alva AS, Aparicio AM, Singal R, Yellapragada S, Sonpavde G, Hahn NM (2011) Epigenetics in prostate cancer. Prostate Cancer 2011:580318
Patra SK, Patra A, Dahiya R (2001) Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochem Biophys Res Commun 287:705–713
Fu M, Rao M, Wang C, Sakamaki T, Wang J, Di Vizio D, Zhang X, Albanese C, Balk S, Chang C, Fan S, Rosen E, Palvimo JJ, Janne OA, Muratoglu S, Avantaggiati ML, Pestell RG (2003) Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol Cell Biol 23:8563–8575
Abbas A, Gupta S (2008) The role of histone deacetylases in prostate cancer. Epigenetics 3:300–309
Walton TJ, Li G, Seth R, McArdle SE, Bishop MC, Rees RC (2008) DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor beta and induce apoptosis in prostate cancer cell-lines. Prostate 68:210–222
Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK (2005) Global histone modification patterns predict risk of prostate cancer recurrence. Nature 435:1262–1266
Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G (2008) Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer 98:604–610
Zhou LX, Li T, Huang YR, Sha JJ, Sun P, Li D (2010) Application of histone modification in the risk prediction of the biochemical recurrence after radical prostatectomy. Asian J Androl 12:171–179
Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, Herger B, Yang Y, Atadja P, Wu J, Bhalla K (2007) Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res 13:4882–4890
Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, Nelson C, Gleave M (2007) Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res 67:10455–10465
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10:537–549
Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM (2000) Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 60:5165–5170
Rokhlin OW, Taghiyev AF, Guseva NV, Glover RA, Chumakov PM, Kravchenko JE, Cohen MB (2005) Androgen regulates apoptosis induced by TNFR family ligands via multiple signaling pathways in LNCaP. Oncogene 24:6773–6784
Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N, Sawyers CL (2009) Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res 69:958–966
Tan J, Zhuang L, Jiang X, Yang KK, Karuturi KM, Yu Q (2006) Apoptosis signal-regulating kinase 1 is a direct target of E2F1 and contributes to histone deacetylase inhibitor-induced apoptosis through positive feedback regulation of E2F1 apoptotic activity. J Biol Chem 281:10508–10515
Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T (2000) Regulation of E2F1 activity by acetylation. EMBO J 19:662–671
Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280:233–241
Prince HM, Bishton MJ, Johnstone RW (2009) Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol 5:601–612
Liu X, Gomez-Pinillos A, Liu X, Johnson EM, Ferrari AC (2010) Induction of bicalutamide sensitivity in prostate cancer cells by an epigenetic Puralpha-mediated decrease in androgen receptor levels. Prostate 70:179–189
Shao W, Growney J, O’Connor G, Feng Y, Scher H, Yao Y, Fawell S, Atadja P (2008) Efficacy of panobinostat (LBH589) in prostate cancer cell models: targeting the androgen receptor in hormone-refractory prostate cancer (HRPC). Genitourinary Cancers Symposium:[abstract 216]
Rathkopf D, Wong BY, Ross RW, Anand A, Tanaka E, Woo MM, Hu J, Dzik-Jurasz A, Yang W, Scher HI (2010) A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 66:181–189
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M, Prostate Cancer Clinical Trials Working Group (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26:1148–1159
Scher HI, Morris MJ, Kelly WK, Schwartz LH, Heller G (2005) Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin Cancer Res 11:5223–5232
Sharma S, Vogelzang NJ, Beck J, Patnaik A, Mita M, Dugan M, Hwang A, Masson E, Culver KW, Prince H (2007) Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of LBH589, a novel deacetylase (DAC) inhibitor given intravenously on a new once weekly schedule. J Clin Oncol 25:[abstract 14019]
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 17:3461–3467
Beekman KW, Fleming MT, Scher HI, Slovin SF, Ishill NM, Heller G, Kelly WK (2005) Second-line chemotherapy for prostate cancer: patient characteristics and survival. Clin Prostate Cancer 4:86–90
Halabi S, Vogelzang NJ, Ou SS, Owzar K, Archer L, Small EJ (2009) Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol 27:2766–2771
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI, COU-AA-301 Investigators (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS, AFFIRM Investigators (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187–1197
Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC, Pili R, Zwiebel J, Scher H, Hussain M (2009) Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer 115:5541–5549
Schrump DS (2009) Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: mechanisms and potential clinical implications. Clin Cancer Res 15:3947–3957
Subramanian S, Bates S, Wright J, Espinoza-Delgado I, Piekarz R (2010) Clinical toxicities of histone deacetylase inhibitors. Pharmaceuticals 3:2751–2767
Acknowledgments
This study was funded by Novartis Pharmaceuticals Corporation. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Kerry K. Brinkman, PhD, for medical editorial assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathkopf, D.E., Picus, J., Hussain, A. et al. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 72, 537–544 (2013). https://doi.org/10.1007/s00280-013-2224-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2224-8