Abstract
Purpose
The objectives of this phase I study were to determine the maximum-tolerated dose (MTD), dose-limiting toxicities (DLTs), and preliminary efficacy of intraperitoneally administered irinotecan (CPT-11) in gastric cancer patients with peritoneal seeding.
Experimental design
Gastric adenocarcinoma patients with surgical biopsy proven peritoneal seeding were enrolled at the time of surgery. Prior to IP chemotherapy, patients underwent palliative gastrectomy and CAPD catheter insertion in which CPT-11 was administered on postoperative day 1. The IP CPT-11 was initiated at 50 mg/m2, which was escalated to 100, 150, 200, 250, and 300 mg/m2. IP CPT-11 chemotherapy was repeated every 3 weeks.
Results
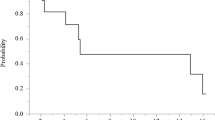
Seventeen patients received a total of 56 cycles at five different CPT-11 dose levels. The DLTs were neutropenic fever, neutropenia, and diarrhea. At the dose level 2 (100 mg/m2), there were one DLTs in one of the first cohort of three patients, but no DLTs at the second cohort of this level. At the dose level 5 (250 mg/m2), two DLTs were detected in the first two patients; thus, the accrual was stopped resulting in the recommended dose of IP CPT-11 of 200 mg/m2. Median progression-free survival was 8.6 months (95% CI, 5.9,11.2), and median overall survival was 15.6 months (95% CI, 8.4,22.8). Pharmacokinetic results of the study showed that the C max of peritoneal SN-38 was achieved earlier than that of plasma SN-38.
Conclusions
Intraperitoneally administered CPT-11 was feasible and tolerable. Further, phase II study of IP CPT-11 in gastric cancer patients with peritoneal seeding is warranted.



Similar content being viewed by others
References
Ajani JA (2005) Evolving chemotherapy for advanced gastric cancer. Oncologist 10(Suppl 3):49–58
Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335:1950–1955
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43
Bouche O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, Arsene D, Paitel JF, Guerin-Meyer V, Mitry E, Buecher B, Kaminsky MC, Seitz JF, Rougier P, Bedenne L, Milan C (2004) Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie digestive group study—FFCD 9803. J Clin Oncol 22:4319–4328
Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, Pignon JP, Drouard-Troalen L, Ouellet JF, Ducreux M (2002) Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 13:267–272
Elias D, Matsuhisa T, Sideris L, Liberale G, Drouard-Troalen L, Raynard B, Pocard M, Puizillou JM, Billard V, Bourget P, Ducreux M (2004) Heated intra-operative intraperitoneal oxaliplatin plus irinotecan after complete resection of peritoneal carcinomatosis: pharmacokinetics, tissue distribution and tolerance. Ann Oncol 15:1558–1565
Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, Francois Y, Vignal J, Gilly FN (2004) Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 139:20–26
Guichard S, Chatelut E, Lochon I, Bugat R, Mahjoubi M, Canal P (1998) Comparison of the pharmacokinetics and efficacy of irinotecan after administration by the intravenous versus intraperitoneal route in mice. Cancer Chemother Pharmacol 42:165–170
Hagiwara A, Takahashi T, Kojima O, Sawai K, Yamaguchi T, Yamane T, Taniguchi H, Kitamura K, Noguchi A, Seiki K et al (1992) Prophylaxis with carbon-adsorbed mitomycin against peritoneal recurrence of gastric cancer. Lancet 339:629–631
Kaneda N, Nagata H, Furuta T, Yokokura T (1990) Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Res 50:1715–1720
Kim ST, Kang WK, Kang JH, Park KW, Lee J, Lee SH, Park JO, Kim K, Kim WS, Jung CW, Park YS, Im YH, Park K (2005) Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer. Br J Cancer 92:1850–1854
Kodera Y, Ito Y, Ito S, Ohashi N, Mochizuki Y, Yamamura Y, Koike M, Fujiwara M, Nakanishi H, Nakao A (2007) Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology 54:960–963
Makrin V, Lev-Chelouche D, Even Sapir E, Paran H, Rabau M, Gutman M (2005) Intraperitoneal heated chemotherapy affects healing of experimental colonic anastomosis: an animal study. J Surg Oncol 89:18–22
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the gynecologic oncology group, Southwestern oncology group, and Eastern cooperative oncology group. J Clin Oncol 19:1001–1007
Maruyama M, Nagahama T, Yuasa Y (1999) Intraperitoneal versus intravenous CPT-11 for peritoneal seeding and liver metastasis. Anticancer Res 19:4187–4191
Maruyama M, Toukairin Y, Baba H, Kure N, Nagahama T, Ebuchi M (2001) Pharmacokinetic study of the intraperitoneal administration of CPT-11 for patients with peritoneal seedings of gastric and colonic cancers. Gan To Kagaku Ryoho 28:1505–1507
Moehler M, Eimermacher A, Siebler J, Hohler T, Wein A, Menges M, Flieger D, Junginger T, Geer T, Gracien E, Galle PR, Heike M (2005) Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer 92:2122–2128
Rosen HR, Jatzko G, Repse S, Potrc S, Neudorfer H, Sandbichler P, Zacherl J, Rabl H, Holzberger P, Lisborg P, Czeijka M (1998) Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian working group for surgical oncology. J Clin Oncol 16:2733–2738
Topuz E, Basaran M, Saip P, Aydiner A, Argon A, Sakar B, Tas F, Uygun K, Bugra D, Aykan NF (2002) Adjuvant intraperitoneal chemotherapy with cisplatinum, mitoxantrone, 5-fluorouracil, and calcium folinate in patients with gastric cancer: a phase II study. Am J Clin Oncol 25:619–624
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21:3737–3743
Yonemura Y, Endou Y, Bando E, Kuno K, Kawamura T, Kimura M, Shimada T, Miyamoto K, Sasaki T, Sugarbaker PH (2004) Effect of intraperitoneal administration of docetaxel on peritoneal dissemination of gastric cancer. Cancer Lett 210:189–196
Author information
Authors and Affiliations
Corresponding author
Additional information
M. K. Choi and B. J. Ahn contributed equally to this study.
Rights and permissions
About this article
Cite this article
Choi, M.K., Ahn, BJ., Yim, DS. et al. Phase I study of intraperitoneal irinotecan in patients with gastric adenocarcinoma with peritoneal seeding. Cancer Chemother Pharmacol 67, 5–11 (2011). https://doi.org/10.1007/s00280-010-1272-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1272-6