Abstract
Purpose
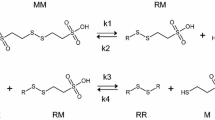
The mechanisms for cisplatin-induced renal cell injury have been the focus of intense investigation for many years with a view to provide a more effective and convenient form of nephroprotection. BNP7787 (disodium 2,2′-dithio-bis ethane sulfonate; dimesna, Tavocept™), is a water-soluble disulfide investigational new drug that is undergoing clinical development for the prevention and mitigation of clinically important chemotherapy-induced toxicities associated with platinum-type chemotherapeutic agents. We hypothesized that part of BNP7787’s mechanism of action (MOA) pertaining to the potential prevention of cisplatin-induced nephrotoxicity involves the inhibition of gamma-glutamyl transpeptidase (GGT) activity, mediated by BNP7787-derived mesna–disulfide heteroconjugates that contain a terminal gamma-glutamate moiety [e.g., mesna–glutathione (MSSGlutathione) and mesna–cysteinyl-glutamate (MSSCE)].
Methods
Inhibition studies were conducted on human and porcine GGT to determine the effect of mesna–disulfide heteroconjugates on the enzyme’s activity in vitro. These studies utilized a fluorimetric assay that monitored the hydrolysis of l-gamma-glutamyl-7-amino-4-trifluoromethylcoumarin (GG-AFC) to AFC.
Results
Mesna–disulfide heteroconjugates that contained gamma-glutamyl moieties were potent inhibitors of human and porcine GGT. An in situ-generated mesna–cisplatin conjugate was not a substrate for GGT.
Conclusions
The GGT xenobiotic metabolism pathway is postulated to be a major toxification pathway for cisplatin nephrotoxicity, and BNP7787 may play a novel and critical therapeutic role in the modulation of GGT activity. We further postulate that there are two general mechanisms for BNP7787-mediated nephroprotection against cisplatin-induced nephrotoxicity involving this pathway. First, the active BNP7787 pharmacophore, mesna, produces an inactive mesna–cisplatin conjugate that is not a substrate for the GGT toxification pathway (GGT xenobiotic metabolism pathway) and, second, BNP7787-derived mesna–disulfide heteroconjugates may serve as selective, potent inhibitors of GGT, possibly resulting in nephroprotection by a novel means.





Similar content being viewed by others
References
Wong E, Giandomenico CM (1999) Current status of platinum-based antitumor drugs. Chem Rev 99:2451–2466
Go RS, Adjei AA (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 17:409–422
Reed E (2006) Cisplatin and analogs. In: Cancer chemotherapy and biotherapy. Chap. 14. Lippincott Williams & Wilkins, Philadelphia, pp 332–343
Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH (2003) Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol 14:1–10
Levi J, Jacobs C, Kalman SM, McTigue M, Weiner MW (1980) Mechanism of cis-platinum nephrotoxicity: I. Effects of sulfhydryl groups in rat kidneys. J Pharmacol Exp Ther 213:545–550
Tanaka H, Ishikawa E, Teshima S, Shimizu E (1986) Histopathological study of human cisplatin nephropathy. Toxicol Pathol 14:247–257
Arany I, Safirstein RL (2003) Cisplatin nephrotoxicity. Semin Nephrol 23:460–464
Daugaard G, Abildgaard U (1989) Cisplatin nephrotoxicity: a review. Cancer Chemother Pharmacol 25:1–9
Gemba M, Fukuishi N (1991) Amelioration by ascorbic acid of cisplatin-induced injury in cultured renal epithelial cells. Contrib Nephrol 95:138–142
Madias NE, Harrington JT (1978) Platinum nephrotoxicity. Am J Med 65:307–314
Meijer S, Sleijfer DT, Mulder NH, Sluiter WJ, Marrink J, Koops HS, Brouwers TM, Oldhoff J, van der Hem GK, Mandema E (1983) Some effects of combination chemotherapy with cis-platinum on renal function in patients with nonseminomatous testicular carcinoma. Cancer 51:2035–2040
Offerman JJ, Meijer S, Sleijfer DT, Mulder NH, Donker AJ, Koops HS, van der Hem GK (1984) Acute effects of cis-diamminedichloroplatinum (CDDP) on renal function. Cancer Chemother Pharmacol 12:36–38
Weiner MW, Jacobs C (1983) Mechanism of cisplatin nephrotoxicity. Fed Proc 42:2974–2978
Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW (1980) Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J Pharmacol Exp Ther 213:551–556
Gonzales-Vitale JC, Hayes DM, Cvitkovic E, Sternberg SS (1977) The renal pathology in clinical trials of cis-platinum (II) diamminedichloride. Cancer 39:1362–1371
Kuhlmann MK, Burkhardt G, Kohler H (1997) Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant 12:2478–2480
Hanigan MH, Gallagher BC, Taylor PT Jr, Large MK (1994) Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res 54:5925–5929
Hanigan MH, Gallagher BC, Taylor PT Jr (1996) Cisplatin nephrotoxicity: inhibition of gamma-glutamyl transpeptidase blocks the nephrotoxicity of cisplatin without reducing platinum concentrations in the kidney. Am J Obstet Gynecol 175:270–274
Zhang L, Hanigan MH (2003) Role of cysteine S-conjugate beta-lyase in the metabolism of cisplatin. J Pharmacol Exp Ther 306:988–994
Hanigan MH, Lykissa ED, Townsend DM, Ou CN, Barrios R, Lieberman MW (2001) Gamma-glutamyl transpeptidase-deficient mice are resistant to the nephrotoxic effects of cisplatin. Am J Pathol 159:1889–1894
Townsend DM, Hanigan MH (2002) Inhibition of gamma-glutamyl transpeptidase or cysteine S-conjugate beta-lyase activity blocks the nephrotoxicity of cisplatin in mice. J Pharmacol Exp Ther 300:142–148
Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME (1992) High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA 89:3070–3074
Hanigan MH, Gallagher BC, Townsend DM, Gabarra V (1999) Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis 20:553–559
Benlloch M, Ortega A, Ferrer P, Segarra R, Obrador E, Asensi M, Carretero J, Estrela JM (2005) Acceleration of glutathione efflux and inhibition of gamma-glutamyl transpeptidase sensitize metastatic B16 melanoma cells to endothelium-induced cytotoxicity. J Biol Chem 280:6950–6959
Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H (2005) Gamma-glutamyl transferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163, 944 Austrian adults. Circulation 112:2130–2137
Paolicchi A, Minotti G, Tonarelli P, Tongiani R, De CD, Mezzetti A, Dominici S, Comporti M, Pompella A (1999) Gamma-glutamyl transpeptidase-dependent iron reduction and LDL oxidation: a potential mechanism in atherosclerosis. J Investig Med 47:151–160
Hanigan MH (1998) Gamma-glutamyl transpeptidase, a glutathionase: its expression and function in carcinogenesis. Chem Biol Interact 111–112:333–342
Hanigan MH, Pitot HC (1985) Gamma-glutamyl transpeptidase: its role in hepatocarcinogenesis. Carcinogenesis 6:165–172
Tate SS, Meister A (1981) Gamma-glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem 39:357–368
Keillor JW, Castonguay R, Lherbet C (2005) Gamma-glutamyl transpeptidase substrate specificity and catalytic mechanism. Methods Enzymol 401:449–467
Deneke SM, Fanburg BL (1989) Regulation of cellular glutathione. Am J Physiol 257:L163–L173
Boven E, Verschraagen M, Hulscher TM, Erkelens CA, Hausheer FH, Pinedo HM, van der Vijgh WJF (2002) BNP7787, a novel protector against platinum-related toxicities, does not affect the efficacy of cisplatin or carboplatin in human tumour xenografts. Eur J Cancer 38:1148–1156
Hausheer FH, Kanter P, Cao S, Haridas K, Seetharamulu P, Reddy D, Petluru P, Zhao M, Murali D, Saxe JD, Yao S, Martinez N, Zukowski A, Rustum YM (1998) Modulation of platinum-induced toxicities and therapeutic index: mechanistic insights and first- and second-generation protecting agents. Semin Oncol 25:584–599
Pendyala L, Schwartz G, Smith P, Zdanowicz J, Murphy M, Hausheer F (2003) Modulation of plasma thiols and mixed disulfides by BNP7787 in patients receiving paclitaxel/cisplatin therapy. Cancer Chemother Pharmacol 51:376–384
Hausheer FH, Kochat H, Parker AR, Ding D, Yao S, Hamilton SE, Petluru PN, Leverett BD, Bain SH, Saxe JD (2003) New approaches to drug discovery and development: a mechanism-based approach to pharmaceutical research and its application to BNP7787, a novel chemoprotective agent. Cancer Chemother Pharmacol 52(Suppl 1):S3–S15
Verschraagen M, Boven E, Ruijter R, van der Born K, Berkhof J, Hausheer FH, van der Vijgh WJF (2003) Pharmacokinetics and preliminary clinical data of the novel chemoprotectant BNP7787 and cisplatin and their metabolites. Clin Pharmacol Ther 74:157–169
Boven E, Westerman M, van Groeningen CJ, Verschraagen M, Ruijter R, Zegers I, van der Vijgh WJF, Giaccone G (2005) Phase I and pharmacokinetic study of the novel chemoprotector BNP7787 in combination with cisplatin and attempt to eliminate the hydration schedule. Br J Cancer 92:1636–1643
Hausheer F, Kanter P, Rustum Y, Cao S, Haridas K, Reddy D, Seetharamulu P, Zhao M, Yao S, Pavankumar P, Murali D (1997) Abstract #2089: BNP7787: A novel antitumor potentiating drug which protects against cisplatin and carboplatin toxicities. In: Proceedings of the AACR (88th annual meeting), vol 38, p 311
Hausheer F, Cavaletti G, Tredici G, Oggioni N, Spinelli S, Pezzoni G, Manzotti C, Haridas K, Reddy D, Zhao M, Seetharamulu P, Yao S, Pavankumar P, Murali D, Wu M, Saxe J, Cavalletti E (1999) Abstract #2633: Oral and intravenous BNP7787 protects against platinum neurotoxicity without in vitro or in vivo tumor protection. In: Proceedings of the AACR (90th annual meeting), vol 40, p 398
Hausheer FH, Rustum Y, Cao S, Haridas K, Reddy D, Seetharamalu P, Zhao M, Yao S, Pavankumar P, Murali D (1998) Abstract #1077: BNP7787—Administration in vivo results in increased therapeutic index and toxicity reduction of platinum drugs. In: Proceedings of the AACR (89th annual meeting), vol 39, p 158
Shanmugarajah D, Ding D, Huang Q, Chen X, Kochat H, Petluru PN, Ayala PY, Parker AR, Hausheer FH (2009) Analysis of BNP7787 thiol-disulfide exchange reactions in phosphate buffer and human plasma using microscale electrochemical high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 877:857–866
Shanmugarajah D (2007) Mechanism of action of BNP7787, a novel chemoprotective agent. Dissertation, presented to the University of Texas Health Science Center at San Antonio
Ormstad K, Uehara N (1982) Renal transport and disposition of Na-2-mercaptoethane sulfonate disulfide (dimesna) in the rat. FEBS Lett 150:354–358
Verschraagen M, Boven E, Torun E, Erkelens CA, Hausheer FH, van der Vijgh WJF (2004) Pharmacokinetic behaviour of the chemoprotectants BNP7787 and mesna after an i.v. bolus injection in rats. Br J Cancer 90:1654–1659
Tate SS, Meister A (1974) Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem 249:7593–7602
Thompson GA, Meister A (1977) Interrelationships between the binding sites for amino acids, dipeptides, and gamma-glutamyl donors in gamma-glutamyl transpeptidase. J Biol Chem 252:6792–6798
Thompson GA, Meister A (1975) Utilization of l-cystine by the gamma-glutamyl transpeptidase–gamma-glutamyl cyclotransferase pathway. Proc Natl Acad Sci USA 72:1985–1988
Forman HJ, Shi MM, Iwamoto T, Liu RM, Robison TW (1995) Measurement of gamma-glutamyl transpeptidase and gamma-glutamylcysteine synthetase activities in cells. Methods Enzymol 252:66–71
Smith GD, Ding JL, Peters TJ (1979) A sensitive fluorimetric assay for gamma-glutamyl transferase. Anal Biochem 100:136–139
Kintzel PE (2001) Anticancer drug-induced kidney disorders. Drug Saf 24:19–38
Jones MM, Basinger MA, Holscher MA (1992) Control of the nephrotoxicity of cisplatin by clinically used sulfur-containing compounds. Fundam Appl Toxicol 18:181–188
Alberts DS, Bleyer WA (1996) Future development of amifostine in cancer treatment. Semin Oncol 23:90–99
Gandara DR, Wiebe VJ, Perez EA, Makuch RW, DeGregorio MW (1990) Cisplatin rescue therapy: experience with sodium thiosulfate, WR2721, and diethyldithiocarbamate. Crit Rev Oncol Hematol 10:353–365
Kemp G, Rose P, Lurain J, Berman M, Manetta A, Roullet B, Homesley H, Belpomme D, Glick J (1996) Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol 14:2101–2112
Verschraagen M, Zwiers TH, de Koning PE, Welink J, van der Vijgh WJF (2001) Quantification of BNP7787 (dimesna) and its metabolite mesna in human plasma and urine by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 753:293–302
Verschraagen M, van der Born K, Zwiers TH, van der Vijgh WJF (2002) Simultaneous determination of intact cisplatin and its metabolite monohydrated cisplatin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 772:273–281
Verschraagen M, Zwiers TH, Torun E, Donker MG, Reinhoud NJ, van der Vijgh WJF (2003) Simultaneous determination of BNP7787 and its metabolite mesna in plasma and tissue by micro-HPLC with a dual electrochemical detector. J Pharm Sci 92:1040–1050
Verschraagen M, Bosma M, Zwiers TH, Torun E, van der Vijgh WJF (2003) Quantification of mesna and total mesna in kidney tissue by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci 783:33–42
Verschraagen M, Kedde MA, Hausheer FH, van der Vijgh WJF (2003) The chemical reactivity of BNP7787 and its metabolite mesna with the cytostatic agent cisplatin: comparison with the nucleophiles thiosulfate, DDTC, glutathione and its disulfide GSSG. Cancer Chemother Pharmacol 51:499–504
Verschraagen M, Boven E, Torun E, Hausheer FH, Bast A, van der Vijgh WJF (2004) Possible (enzymatic) routes and biological sites for metabolic reduction of BNP7787, a new protector against cisplatin-induced side-effects. Biochem Pharmacol 68:493–502
Hausheer FH, Kochat H, Reddy D, Zhao M, Seetharamulu P, Yao S, Pavankumar P, Murali D, Wu M, Saxe J, Parker A, Hamilton S (2000) Abstract #4890: BNP7787: A novel chemoprotecting agent for platinum and taxane toxicity. In: Proceedings of the AACR (91st annual meeting), vol 41, pp 769–770
Hausheer FH, Kochat H, Zhao M, Hamilton S, Wu M, Seetharamulu P, Petluru P, Huang Q, Chen X, Ma H, Ding D, Leverett B, Wu Y, Wang J, Saxe J, Parker A, Berghorn E (2001) Abstract #1990: BNP7787, A novel neuroprotective agent in taxane and platinum regimens, does not interfere with antitumor activity. In: Proceedings of the AACR (92nd annual meeting), vol 42, p 370
Anders MW, Dekant W (1998) Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol 38:501–537
Dekant W (1993) Bioactivation of nephrotoxins and renal carcinogens by glutathione S-conjugate formation. Toxicol Lett 67:151–160
Dekant W, Vamvakas S, Anders MW (1994) Formation and fate of nephrotoxic and cytotoxic glutathione S-conjugates: cysteine conjugate beta-lyase pathway. Adv Pharmacol 27:115–162
Dekant W, Henschler D (1999) Organ-specific carcinogenicity of haloalkenes mediated by glutathione conjugation. J Cancer Res Clin Oncol 125:174–181
Dekant W (2001) Chemical-induced nephrotoxicity mediated by glutathione S-conjugate formation. Toxicol Lett 124:21–36
Okada T, Suzuki H, Wada K, Kumagai H, Fukuyama K (2007) Crystal structure of the gamma-glutamyl transpeptidase precursor protein from Escherichia coli: structural changes upon autocatalytic processing and implications for the maturation mechanism. J Biol Chem 282:2433–2439
Okada T, Suzuki H, Wada K, Kumagai H, Fukuyama K (2006) Crystal structures of gamma-glutamyl transpeptidase from Escherichia coli, a key enzyme in glutathione metabolism, and its reaction intermediate. Proc Natl Acad Sci USA 103:6471–6476
Andersson A, Isaksson A, Brattstrom L, Hultberg B (1993) Homocysteine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization. Clin Chem 39:1590–1597
Kleinman WA, Richie JP Jr (2000) Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol 60:19–29
Shaw IC, Graham MI (1987) Mesna: a short review. Cancer Treat Rev 14:67–86
Acknowledgments
We thank Vanessa Sandoval and Erika Ramirez for editorial assistance in the preparation of this manuscript. In addition, we appreciate the time and effort of David Margrave and Dr. Scott Whitaker in reviewing these manuscripts and we thank Julie Martin for providing Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hausheer, F.H., Shanmugarajah, D., Leverett, B.D. et al. Mechanistic study of BNP7787-mediated cisplatin nephroprotection: modulation of gamma-glutamyl transpeptidase. Cancer Chemother Pharmacol 65, 941–951 (2010). https://doi.org/10.1007/s00280-009-1101-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1101-y