Abstract
Purpose
To characterize the stability, pharmacokinetics and metabolism of analogs of gossypol, apogossypol and apogossypol hexaacetate to provide a basis for comparison.
Methods
Gossypol, apogossypol and apogossypol hexaacetate were incubated in plasma or liver microsomes from various species, or administered to mice, respectively, from which the stability, metabolism and pharmacokinetic profiles of these analogs were quantitatively determined using a liquid chromatography-mass spectrometry (LC/MS/MS) method.
Results
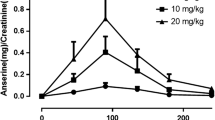
In various species of plasma, apogossypol and gossypol exhibited similar stability, while 20–40% of apogossypol hexaacetate was converted into apogossypol with concurrent formation of the corresponding di-, tri-, tetra-, and penta-acetates of apogossypol. (±)-Gossypol and (−)-gossypol showed comparable pharmacokinetic profile and oral bioavailability (12.2–17.6%) with some variations of clearance and V ss following oral and intravenous administration to mice. At the same molar dose, apogossypol showed delayed T max(1 h), a slower clearance rate and less distribution after administration to mice. Mono- and di-glucuronide conjugates of apogossypol were readily observed in mouse plasma following administration. Apogossypol formulated in sesame oil appeared to possess larger AUC and thus higher oral bioavailability than that formulated in cremophor EL:ethanol:saline. In contrast, intravenous apogossypol hexaacetate exhibited highest clearance rate partially due to its conversion into apogossypol. Concomitant with disappearance of apogossypol hexaacetate (iv), apogossypol converted from apogossypol hexaacetate was quantitatively detected, and accounted for ∼30% of total plasma apogossypol hexaacetate. Oral apogossypol hexaacetate showed no bioavailability with little apogossypol occurring in the plasma. In human and mouse liver microsomes, glucuronide conjugates of apogossypol and its acetates were readily identified with the exception of gossypol glucuronidation. Apogossypol appeared more stable in human and mouse liver microsomal preparations than gossypol and apogossypol hexaacetate.
Conclusions
Apogossypol and gossypol show similar oral and intravenous pharmacokinetic profiles and in vitro stability although apogossypol appears to have a slower clearance rate, larger AUC, and better microsomal stability. Apogossypol hexaacetate converts to apogossypol in both in vitro and in vivo settings and lacks any quantifiable oral bioavailability.





Similar content being viewed by others
References
Withers WA, Carruth FE (1915) Gossypol, the toxic substance in cotton-seed meal. J Agri Res 5:261–268
Adams R, Geissman TA, Edwards JD (1960) Gossypol, a pigment of cottonseed. Chem Rev 60:555–574
Waites GM, Wang C, Griffin PD (1998) Gossypol: reasons for its failure to be accepted as a safe, reversible male antifertility drug. Int J Androl 21:8–12
Qian SZ, Wang ZG (1998) Gossypol: a potential antifertility agent for males. (Review). Ann Rev Pharm Toxicol 24:329–360
Matlin SA, Zhou RH (1984) Resolution of gossypol: analytical and preparative HPLC. J High Resol Chromatogr Commun 7:629–631
Matlin SA, Zhou RH, Belenguer A. Tyson RG, Brookes AN (1988) Large-scale resolution of gossypol enantiomers for biological evaluation. Contraception 37:229–237
Radloff RJ, Deck LM, Royer RE, Vander Jagt DL (1986) Antiviral activities of gossypol and its derivatives against herpes simplex virus type II. Pharmacol Res Commun 18:1063–1073
Montamat EE, Burgos C, Gerez de Burgos NM, Rovai LE, Blanco A, Segura EL (1982) Inhibitory action of gossypol on enzymes and growth of Trypanosoma cruzi. Science 218:288–289
Benhaim P, Mathes SJ, Hunt TK, Scheuenstuhl H, Benz CC (1994) Induction of neutrophils Mac-1 integrin expression and superoxide production by the medicinal plant extract gossypol. Inflammation 18:443–458
Bushunow P, Reidenberg MM, Wasenko J et al. (1999) Gossypol treatment of recurrent adult malignant gliomas. J Neuro-Oncol. 43:79–86
Van Poznak C, Seidman AD, Reidenberg MM et al. (2001) Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat 66:239–248
Tuszynski GP, Cossu G (1984) Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res 44:768–771
Wu YW, Chik CL, Knazek RA (1989) An in vitro and in vivo study of antitumor effects of gossypol on human SW-13 adenocortical carcinoma. Cancer Res 49:3753–3758
Coyle T, Levante S, Shetler M, Winfield J (1994) In vitro and in vivo cytotoxicity of gossypol against central nervous system tumor cell lines. J Neuro-Oncol. 19:25–35
Reed JC (2002) Apoptosis-based therapies. Nat Rev Drug Discov 1:111–121
Tso WW, Lee CS (1982) Lactate dehydrogenase-X: an isozyme particularly sensitive to gossypol inhibition. Internat J Androl 5:205–209
Ligueros M, Jeoung D, Hochhauser D, Reidenberg MM, Sonenberg M (1997) Gossypol inhibition of mitosis, cyclin D1 and Rb protein inhuman mammary cancer cells and cyclin D1 transfected human fibrosarcoma cells. Br J Cancer 76:21–28
Shidaifat F, Canatan H, Kulp S et al. (1997) Gossypol arrests human benign prostatic hyperplastic cell growth at Go/G1 phase of the cell cycle. Anticancer Res 17:1003–1009
Teng CS (1995) Gossypol-induced apoptotic DNA fragmentation correlates with inhibited protein kinase C activity in spermatocytes. Contraception 52:389–395
Kitada S, Leone M, Sareth S, Zhai D, Reed J, Pellecchia M (2003) Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem 64:4259–4264
Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, Kipps TJ, Reed JC, Pellecchia M (2004) Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-XL. Chem Biol 11:389–395
Reed JC, Pellecchia M (2005) Apoptosis-based therapies for hematologic malignancies. Blood 106:408–418
Zhai D, Jin C, Satterthwait AC, Reed JC (2006) Comparison of chemical inhibitors of antiapoptotic Bcl-2-familiy proteins. Cell Death Differ 13:1419–1421
Coward LC, Kerstner-Wood, Noker P, Gorman G, Pellecchia M, Reed JC, Jia L (2006) Quantitative determination of Apogossypol, a pro-apoptotic analog of gossypol, in mouse plasma using LC/MS/MS. J Pharm Biomed Anal 42:581–586
Jia L, Noker PE, Coward L, Gorman SG, Protopopova M, Tomaszewski JE (2006) Interspecies pharmacokinetics and in vitro metabolism of SQ109. Br J Pharmacol 147:476–485
Wu DF, Yu YW, Tang ZM, Wang MZ (1986) Pharmacokinetics of (±), (+)- and (−)-gossypol in human and dogs. Clin Pharmacol Ther 39:613–618
Lin YC, Nuber DC, Gu Y, Cutler G, Hinchcliff KW, Haibel G (1991) Gossypol pharmacokinetics in mid-lactation Brown Swiss dairy cows. Vet Res Commun 15:379–385
Kalla NR, Sud S (1990) Distribution of gossypol. Acta Eur Fertil 21:77–80
Abou-Donia MB, Othman MA, Obih P (1989) Interspecies comparison of pharmacokinetic profile and bioavailability of (±)-gossypol in male Fischer-344 rats and male B6C3F mice. Toxicology 55:37–51
Othman MA, Abou-Donia MB (1988) Pharmacokinetic profile of (±)-gossypol in male Sprague-Dawley rats following single intravenous and oral and subchronic oral administration. Proc Soc Exp Biol Med 188:17–22
Parkinson A (1996) Biotransformation of xenobiotics. In: Klaassen C (ed) Casarett and Doull’s toxicology: The basic science of poisons. McGraw-Hill, New York, pp 113–186
Jia L, Wong H, Wang Y, Garza M, Weitman SD (2003) Carbendazim: disposition, cellular permeability, metabolite identification and pharmacokinetic comparison with its nanoparticle. J Pharm Sci 92:161–172
Clark EP (1928) Studies on gossypol. IV. Apogossypol. J Biol Chem 78:159–165
Cater CM, Lyman CM (1996) Reaction of gossypol with amino acids and other amino compounds. J Am Oil Chem Soc 64:649–653
Fish RG, Groundwater PW, Morgan JJG (1995) The photoepimerization of gossypol Schiff’s bases. Tetrahedron Asymmetry 6:873–876
Medrano FJ, Andreu JM (1986) Binding of gossypol to purified tubulin and inhibition of its assembly into microtubules. Eur J Biochem 158:63–69
Appu Rao AG (1992) A stoichiometric analysis of bovine serum albumin-gossypol interactions: a fluorescence quenching study. Indian J Biochem Biophys 29:179–182
Sonenberg M, Huang JT, Ren YF et al. (1988) Anti-fertility and other actions of gossypol analogues. Contraception 37:247–255
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support: This work was supported by Contract NO1-CM-07110 and NO1-CM-52203 from the National Cancer Institute to the Rapid Access to Intervention Development Program (RAID).
Rights and permissions
About this article
Cite this article
Jia, L., Coward, L.C., Kerstner-Wood, C.D. et al. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemother Pharmacol 61, 63–73 (2008). https://doi.org/10.1007/s00280-007-0446-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0446-3