Abstract
Aim
MEN-10755 is a novel anthracycline analogue that has shown an improved therapeutic efficacy over doxorubicin in animal models, especially in gynaecological and lung cancers and is currently under clinical development for the treatment of solid tumours. The aim of the project was to develop an optimal sampling strategy for MEN-10755 to provide an efficient basis for future pharmacokinetic/pharmacodynamic investigations.
Methods
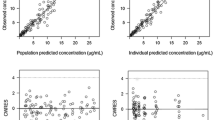
Data from 24 patients who participated in a phase I clinical pharmacokinetic study of MEN-10755 administered as a short i.v. infusion were included. Individual pharmacokinetic values were calculated by fitting the plasma concentration data to a two-compartment model using nonlinear least-squared regression (KINFIT, Ed 3.5). Population pharmacokinetic analysis was carried out using (a) the traditional standard two-stage method (STS) based on all data (KINFIT-ALL), (b) the iterative two-stage Bayesian (IT2B) population modelling algorithm (KINPOP), and (c) the STS method using KINFIT and using four optimally timed plasma concentrations (KINFIT-OSS4). Determinant (D) optimal sampling strategy (OSS) was used to evaluate the four most information-rich sampling times. The pharmacokinetic parameters Vc (l), kel (h−1), k12 (h−1) and k21 (h−1) calculated using KINPOP served as a model for calculation of four D-optimal sampling times. D-optimal sampling data sets were analysed using KINFIT-OSS4 and compared with the population model obtained by the traditional standard two-stage approach for all data sets (KINFIT-ALL).
Results
The optimal sampling times were: the end of the infusion, and 1.5 h, 3.8 h and 24 h after the start of the infusion. The four-point D-optimal sampling design determined in this study gave individual parameter estimates close to the basic standard estimates using the full data set.
Conclusion
Because accurate estimates of pharmacokinetic parameters were achieved, the four-point D-optimal sampling design may be very useful in future studies with MEN-10755.

Similar content being viewed by others
References
Akaike H (1976) An information criterion. Math Sci 14:59
Arcamone F, Animati F, Berettoni M, Bigioni M, Capranico G, Casazza AM, Caserini C, Cipollone A, De Cesare M, Franciotti M, Lombardi P, Madami A, Manzini S, Monteagudo E, Polizzi D, Pratesi G, Righetti SC, Salvatore C, Supino R, Zunino F (1997) Doxorubicin disaccharide analogue: apoptosis-related improvement of efficacy in vivo. J Natl Cancer Inst 89:1217–1223
Bos AM, de Vries EG, Dombernovsky P, Aamdal S, Uges DR, Schrijvers D, Wanders J, Roelvink MW, Hanauske AR, Bortini S, Capriati A, Crea AE, Vermorken JB (2001) Pharmacokinetics of MEN-10755, a novel anthracycline disaccharide analogue, in two phase I studies in adults with advanced solid tumours. Cancer Chemother Pharmacol 48:361–369
Canal P, Chatelut E, Guichard S (1998) Practical treatment guide for dose individualisation in cancer chemotherapy. Drugs 56:1019–1038
Chatelut E, Pivot X, Otto J, Chevreau C, Thyss A, Renee N, Milano G, Canal P (2000) A limited sampling strategy for determining carboplatin AUC and monitoring drug dosage. Eur J Cancer 36:264–269
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Collins JM, Zaharko DS, Dedrick RL, Chabner BA (1986) Potential roles for preclinical pharmacology in phase I clinical trials. Cancer Treat Rep 70:73–80
D’Argenio DZ (1981) Optimal sampling times for pharmacokinetic experiments. J Pharmacokinet Biopharm 9:739–756
D’Argenio DZ, Schumitzky A (1979) A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed 9:115–134
D’Argenio D, Schumitzky A (1992) ADAPT II users guide. Biomedical Simulations Resource, University of Southern California, Los Angeles
Drusano GL (1991) Optimal sampling theory and population modelling: application to determination of the influence of the microgravity environment on drug distribution and elimination. J Clin Pharmacol 31:962–967
Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known, 1916. Nutrition 5:303–311
Egorin MJ, Forrest A, Belani CP, Ratain MJ, Abrams JS, Van Echo DA (1989) A limited sampling strategy for cyclophosphamide pharmacokinetics. Cancer Res 49:3129–3133
EORTC Pharmacokinetics and Metabolism Group (1987) Pharmacokinetically guided dose escalation in phase I clinical trials. Commentary and proposed guidelines. Eur J Cancer Clin Oncol 23:1083–1087
Evans WE, Relling MV (1989) Clinical pharmacokinetics-pharmacodynamics of anticancer drugs. Clin Pharmacokinet 16:327–336
Hon YY, Evans WE (1998) Making TDM work to optimize cancer chemotherapy: a multidisciplinary team approach. Clin Chem 44:388–400
Hortobagyi GN (1997) Anthracyclines in the treatment of cancer. An overview. Drugs 54 [Suppl 4]:1–7
Jelliffe RW, Iglesias T, Hurst AK, Foo KA, Rodriguez J (1991) Individualising gentamicin dosage regimens. A comparative review of selected models, data fitting methods and monitoring strategies. Clin Pharmacokinet 21:461–478
Jelliffe RW, Schumitzky A, Van Guilder M, Liu M, Hu L, Maire P, Gomis P, Barbaut X, Tahani B (1993) Individualizing drug dosage regimens: roles of population pharmacokinetic and dynamic models, Bayesian fitting, and adaptive control. Ther Drug Monit 15:380–393
Jelliffe R, Schumitzky A, Van Guilder M (1994) User manual for version 10.1 of the USC*PACK collection of PC programs. Laboratory of Applied Pharmacokinetics, University of Southern California, School of Medicine, Los Angeles
Jodrell DI, Egorin MJ, Canetta RM, Langenberg P, Goldbloom EP, Burroughs JN, Goodlow JL, Tan S, Wiltshaw E (1992) Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol 10:520–528
Jodrell DI, Murray LS, Hawtof J, Graham MA, Egorin MJ (1996) A comparison of methods for limited-sampling strategy design using data from a phase I trial of the anthrapyrazole DuP-941. Cancer Chemother Pharmacol 37:356–362
Launay MC, Milano G, Iliadis A, Frenay M, Namer N (1989) A limited sampling procedure for estimating adriamycin pharmacokinetics in cancer patients. Br J Cancer 60:89–92
Masson E, Zamboni WC (1997) Pharmacokinetic optimisation of cancer chemotherapy. Effect on outcomes. Clin Pharmacokinet 32:324–343
Mick R, Gupta E, Vokes EE, Ratain MJ (1996) Limited-sampling models for irinotecan pharmacokinetics-pharmacodynamics: prediction of biliary index and intestinal toxicity. J Clin Oncol 14:2012–2019
Minami H, Beijnen JH, Verweij J, Ratain MJ (1996) Limited sampling model for area under the concentration time curve of total topotecan. Clin Cancer Res 2:43–46
Miyazaki M, Fujiwara Y, Takahashi T, Isobe T, Ohune T, Tsuya T, Yamakido M (1997) Limited-sampling models for estimation of the carboplatin area under the curve. Anticancer Res 17:4571–4575
Moore MJ, Erlichman C (1987) Therapeutic drug monitoring in oncology. Problems and potential in antineoplastic therapy. Clin Pharmacokinet 13:205–227
Pratesi G, De Cesare M, Caserini C, Perego P, Dal Bo L, Polizzi D, Supino R, Bigioni M, Manzini S, Iafrate E, Salvatore C, Casazza A, Arcamone F, Zunino F (1998) Improved efficacy and enlarged spectrum of activity of a novel anthracycline disaccharide analogue of doxorubicin against human tumor xenografts. Clin Cancer Res 4:2833–2839
Proost JH, Meijer DK (1992) MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 22:155–163
Ranson MR, Scarffe JH (1994) Population and Bayesian pharmacokinetics in oncology. Clin Oncol (R Coll Radiol) 6:254–260
Ratain MJ, Robert J, van der Vijgh WJ (1991) Limited sampling models for doxorubicin pharmacokinetics. J Clin Oncol 9:871–876
Rousseau A, Marquet P, Debord J, Sabot C, Lachatre G (2000) Adaptive control methods for the dose individualisation of anticancer agents. Clin Pharmacokinet 38:315–353
Schrijvers D, Bos AME, Dyck J, de Vries EGE, Wanders J, Roelvink M, Fumoleau P, Bortini S, Vermorken JB (2002) A phase I study of MEN-10755, a new anthracycline in patients with solid tumours: a report from the European Organization for Research and Treatment of Cancer, Early Clinical Studies Group. Ann Oncol 13:385–391
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Sorensen BT, Stromgren A, Jakobsen P, Jakobsen A (1993) A limited sampling method for estimation of the carboplatin area under the curve. Cancer Chemother Pharmacol 31:324–327
Steimer J, Mallet A, Mentre F (1985) Estimating interindividual pharmacokinetic variability. Variability in drug therapy: description, estimation and control. Raven Press, New York, pp 65–109
Stromgren AS, Sorensen BT, Jakobsen P, Jakobsen A (1993) A limited sampling method for estimation of the etoposide area under the curve. Cancer Chemother Pharmacol 32:226–230
Tranchand B, Amsellem C, Chatelut E, Freyer G, Iliadis A, Ligneau B, Trillet-Lenoir V, Canal P, Lochon I, Ardiet CJ (1999) A limited-sampling strategy for estimation of etoposide pharmacokinetics in cancer patients. Cancer Chemother Pharmacol 43:316–322
van Warmerdam LJ, Bokkel Huinink WW, Maes RA, Beijnen JH (1994) Limited-sampling models for anticancer agents. J Cancer Res Clin Oncol 120:427–433
Vinks AA, Mouton JW, Touw DJ, Heijerman HG, Danhof M, Bakker W (1996) Population pharmacokinetics of ceftazidime in cystic fibrosis patients analyzed by using a nonparametric algorithm and optimal sampling strategy. Antimicrob Agents Chemother 40:1091–1097
Weiss RB (1992) The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 19:670–686
Acknowledgement
The authors are grateful to J.H. Proost (PhD) of the University Centre of Pharmacy in Groningen for his assistance in using the MW\Pharm computer program for pharmacokinetic analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by Menarini Ricerche S.p.A.
Rights and permissions
About this article
Cite this article
Bos, A.M.E., Boom, K., Vinks, A.A. et al. Development of an optimal sampling strategy for clinical pharmacokinetic studies of the novel anthracycline disaccharide analogue MEN-10755. Cancer Chemother Pharmacol 54, 64–70 (2004). https://doi.org/10.1007/s00280-004-0772-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0772-7