Abstract
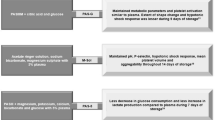
Platelet-rich plasma (PRP) has significant potential for various applications and holds clinical value in regenerative medicine. Cryopreservation is used to extend the preservation period of PRP, facilitating its clinical application. However, the potential negative effects of long-term cryopreservation on platelet storage lesion are still uncertain. In this study, PRP was stored at − 30 °C or − 80 °C. Platelet count, apoptosis, reactive oxygen species (ROS) content, and CD62P expression were assessed on the 14th and 28th days. The study also evaluated platelet mitochondria morphology and function, serotonin (5-HT) secretion by platelets, and the inflammatory activating effect of cryopreserved platelets in PRP. The results showed that there were no significant differences in platelet count, the content of 5-HT, and inflammatory effects between fresh PRP and PRP cryopreserved at both − 30 °C and − 80 °C. However, there was an increase in ROS level, apoptosis, and CD62P level after cryopreservation at both temperatures. Additionally, the levels of ROS, apoptosis, and CD62P in platelets were similar after storage at − 30 °C and − 80 °C. The main difference observed was that the morphology and function of mitochondria were severely damaged after storage at − 30 °C, while they were less affected at − 80 °C. Based on these findings, it can be concluded that storing PRP at − 80 °C is more suitable for achieving a better therapeutic effect in clinical applications, but cryopreservation could not replace the current standard.




Similar content being viewed by others
Availability of data and materials
All data generated during this study are included in this published article. Requests for materials should be addressed to the corresponding author.
References
Pelletier MH, Malhotra A, Brighton T, Walsh WR, Lindeman R (2013) Platelet function and constituents of platelet rich plasma. Int J Sports Med 34(1):74–80. https://doi.org/10.1055/s-0032-1316319
Andia I, Abate M (2013) Platelet-rich plasma: underlying biology and clinical correlates. Regen Med 8(5):645–658. https://doi.org/10.2217/rme.13.59
Shin MK, Lee JW, Kim YI, Kim YO, Seok H, Kim NI (2014) The effects of platelet-rich clot releasate on the expression of MMP-1 and type I collagen in human adult dermal fibroblasts: PRP is a stronger MMP-1 stimulator. Mol Biol Rep 41(1):3–8. https://doi.org/10.1007/s11033-013-2718-9
Anitua E, Pino A, Orive G (2016) Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J Wound Care 25(11):680–687. https://doi.org/10.12968/jowc.2016.25.11.680
Guszczyn T, Surażyński A, Zaręba I, Rysiak E, Popko J, Pałka J (2017) Differential effect of platelet-rich plasma fractions on β1-integrin signaling, collagen biosynthesis, and prolidase activity in human skin fibroblasts. Drug Des Devel Ther 11:1849–1857. https://doi.org/10.2147/dddt.S135949
Shiga Y, Kubota G, Orita S, Inage K, Kamoda H, Yamashita M, Iseki T, Ito M, Yamauchi K, Eguchi Y, Sainoh T, Sato J, Fujimoto K, Abe K, Kanamoto H, Inoue M, Kinoshita H, Furuya T, Koda M, Aoki Y, Toyone T, Takahashi K, Ohtori S (2017) Freeze-dried human platelet-rich plasma retains activation and growth factor expression after an eight-week preservation period. Asian Spine J 11(3):329–336. https://doi.org/10.4184/asj.2017.11.3.329
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci 21(20):7794. https://doi.org/10.3390/ijms21207794
Sharara FI, Lelea LL, Rahman S, Klebanoff JS, Moawad GN (2021) A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J Assist Reprod Genet 38(5):1003–1012. https://doi.org/10.1007/s10815-021-02146-9
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE (2019) OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 27(11):1578–1589. https://doi.org/10.1016/j.joca.2019.06.011
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, Gellar K, Harvey WF, Hawker G, Herzig E, Kwoh CK, Nelson AE, Samuels J, Scanzello C, White D, Wise B, Altman RD, DiRenzo D, Fontanarosa J, Giradi G, Ishimori M, Misra D, Shah AA, Shmagel AK, Thoma LM, Turgunbaev M, Turner AS, Reston J (2020) 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 72(2):220–233. https://doi.org/10.1002/art.41142
Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A, Harris A, Yu SP, Connell D, Linklater J, Wang BH, Oo WM, Hunter DJ (2021) Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA 326(20):2021–2030. https://doi.org/10.1001/jama.2021.19415
Peng YN, Chen JL, Hsu CC, Chen CPC, Suputtitada A (2022) Intra-articular leukocyte-rich platelet-rich plasma versus intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis of 14 randomized controlled trials. Pharmaceuticals (Basel) 15(8):974. https://doi.org/10.3390/ph15080974
Paget LDA, Reurink G, de Vos RJ, Weir A, Moen MH, Bierma-Zeinstra SMA, Stufkens SAS, Goedegebuure S, Krips R, Maas M, Meuffels DE, Nolte PA, Runhaar J, Kerkhoffs G, Tol JL (2023) Platelet-rich plasma injections for the treatment of ankle osteoarthritis. Am J Sports Med 51(10):2625–2634. https://doi.org/10.1177/03635465231182438
Verma R, Kumar S, Garg P, Verma YK (2023) Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank 24(2):285–306. https://doi.org/10.1007/s10561-022-10039-z
Tischer T, Bode G, Buhs M, Marquass B, Nehrer S, Vogt S, Zinser W, Angele P, Spahn G, Welsch GH, Niemeyer P, Madry H (2020) Platelet-rich plasma (PRP) as therapy for cartilage, tendon and muscle damage - German working group position statement. J Exp Orthop 7(1):64. https://doi.org/10.1186/s40634-020-00282-2
Kim JI, Bae HC, Park HJ, Lee MC, Han HS (2020) Effect of storage conditions and activation on growth factor concentration in platelet-rich plasma. J Orthop Res 38(4):777–784. https://doi.org/10.1002/jor.24520
Lai H, Chen G, Zhang W, Wu G, Xia Z (2023) Research trends on platelet-rich plasma in the treatment of wounds during 2002–2021: a 20-year bibliometric analysis. Int Wound J 20(6):1882–1892. https://doi.org/10.1111/iwj.14047
Kikuchi N, Yoshioka T, Taniguchi Y, Sugaya H, Arai N, Kanamori A, Yamazaki M (2019) Optimization of leukocyte-poor platelet-rich plasma preparation: a validation study of leukocyte-poor platelet-rich plasma obtained using different preparer, storage, and activation methods. J Exp Orthop 6(1):24. https://doi.org/10.1186/s40634-019-0190-8
Kinoshita H, Orita S, Inage K, Fujimoto K, Shiga Y, Abe K, Inoue M, Norimoto M, Umimura T, Ishii T, Yonemoto T, Kamoda H, Tsukanishi T, Suzuki M, Hirosawa N, Akazawa T, Ohtori S (2020) Freeze-dried platelet-rich plasma induces osteoblast proliferation via platelet-derived growth factor receptor-mediated signal transduction. Asian Spine J 14(1):1–8. https://doi.org/10.31616/asj.2019.0048
Whitney KE, Dornan GJ, King J, Chahla J, Evans TA, Philippon MJ, LaPrade RF, Huard J (2021) The effect of a single freeze-thaw cycle on matrix metalloproteinases in different human platelet-rich plasma formulations. Biomedicines 9(10):1403. https://doi.org/10.3390/biomedicines9101403
White JG, Hagert K, Nipper JH, Rao GH (1980) Functional platelets after storage in vitro for 15–21 days. Am J Pathol 101(3):613–634
Murphy S, Gardner FH (1969) Effect of storage temperature on maintenance of platelet viability–deleterious effect of refrigerated storage. N Engl J Med 280(20):1094–1098. https://doi.org/10.1056/nejm196905152802004
Beitia M, Delgado D, Mercader J, Gimeno I, Espregueira-Mendes J, Aizpurua B, Sánchez M (2023) The effect of short-term cryopreservation on the properties and functionality of platelet-rich plasma. Platelets 34(1):2210243. https://doi.org/10.1080/09537104.2023.2210243
Cecerska-Heryć E, Goszka M, Serwin N, Roszak M, Grygorcewicz B, Heryć R, Dołęgowska B (2022) Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev 64:84–94. https://doi.org/10.1016/j.cytogfr.2021.11.003
Jin P, Pan Q, Lin Y, Dong Y, Zhu J, Liu T, Zhu W, Cheng B (2023) Platelets facilitate wound healing by mitochondrial transfer and reducing oxidative stress in endothelial cells. Oxid Med Cell Longev 2023:2345279. https://doi.org/10.1155/2023/2345279
Kim S, Kim Y, Yu SH, Lee SE, Park JH, Cho G, Choi C, Han K, Kim CH, Kang YC (2022) Platelet-derived mitochondria transfer facilitates wound-closure by modulating ROS levels in dermal fibroblasts. Platelets 34(1):2151996. https://doi.org/10.1080/09537104.2022.2151996
Mammadova-Bach E, Mauler M, Braun A, Duerschmied D (2018) Autocrine and paracrine regulatory functions of platelet serotonin. Platelets 29(6):541–548. https://doi.org/10.1080/09537104.2018.1478072
Mezger M, Nording H, Sauter R, Graf T, Heim C, von Bubnoff N, Ensminger SM, Langer HF (2019) Platelets and immune responses during thromboinflammation. Front Immunol 10:1731. https://doi.org/10.3389/fimmu.2019.01731
Roffi A, Filardo G, Assirelli E, Cavallo C, Cenacchi A, Facchini A, Grigolo B, Kon E, Mariani E, Pratelli L, Pulsatelli L, Marcacci M (2014) Does platelet-rich plasma freeze-thawing influence growth factor release and their effects on chondrocytes and synoviocytes? Biomed Res Int 2014:692913. https://doi.org/10.1155/2014/692913
Fukuda K, Kuroda T, Tamura N, Mita H, Kasashima Y (2020) Optimal activation methods for maximizing the concentrations of platelet-derived growth factor-BB and transforming growth factor-β1 in equine platelet-rich plasma. J Vet Med Sci 82(10):1472–1479. https://doi.org/10.1292/jvms.20-0167
Anitua E, de la Fuente M, Muruzábal F, Merayo-Lloves J (2021) Stability of freeze-dried plasma rich in growth factors eye drops stored for 3 months at different temperature conditions. Eur J Ophthalmol 31(2):354–360. https://doi.org/10.1177/1120672120913035
Melnikov DV, Kirillova KA, Zakharenko AS, Sinelnikov MY, Ragimov AA, Istranov AL, Startseva OI (2021) Effect of cryo-processing on platelet-rich autoplasma preparations. Sovrem Tekhnologii Med 12(6):54–60. https://doi.org/10.17691/stm2020.12.6.07
Green ID, Pinello N, Song R, Lee Q, Halstead JM, Kwok CT, Wong ACH, Nair SS, Clark SJ, Roediger B, Schmitz U, Larance M, Hayashi R, Rasko JEJ, Wong JJ (2020) Macrophage development and activation involve coordinated intron retention in key inflammatory regulators. Nucleic Acids Res 48(12):6513–6529. https://doi.org/10.1093/nar/gkaa435
Masselli E, Pozzi G, Vaccarezza M, Mirandola P, Galli D, Vitale M, Carubbi C, Gobbi G (2020) ROS in platelet biology: functional aspects and methodological insights. Int J Mol Sci 21(14):4866. https://doi.org/10.3390/ijms21144866
Wang SB, Jang JY, Chae YH, Min JH, Baek JY, Kim M, Park Y, Hwang GS, Ryu JS, Chang TS (2015) Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic Biol Med 83:41–53. https://doi.org/10.1016/j.freeradbiomed.2015.01.018
Delaney MK, Kim K, Estevez B, Xu Z, Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J, Du X (2016) Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol 36(5):846–854. https://doi.org/10.1161/atvbaha.116.307308
Ghasemzadeh M, Hosseini E, Roudsari ZO, Zadkhak P (2018) Intraplatelet reactive oxygen species (ROS) correlate with the shedding of adhesive receptors, microvesiculation and platelet adhesion to collagen during storage: does endogenous ROS generation downregulate platelet adhesive function? Thromb Res 163:153–161. https://doi.org/10.1016/j.thromres.2018.01.048
Hosseini E, Ghasemzadeh M, Nassaji F, Jamaat ZP (2017) GPVI modulation during platelet activation and storage: its expression levels and ectodomain shedding compared to markers of platelet storage lesion. Platelets 28(5):498–508. https://doi.org/10.1080/09537104.2016.1235692
Six KR, Compernolle V, Feys HB (2020) Platelet biochemistry and morphology after cryopreservation. Int J Mol Sci 21(3):935. https://doi.org/10.3390/ijms21030935
Marks DC, Johnson L (2019) Assays for phenotypic and functional characterization of cryopreserved platelets. Platelets 30(1):48–55. https://doi.org/10.1080/09537104.2018.1514108
Levoux J, Prola A, Lafuste P, Gervais M, Chevallier N, Koumaiha Z, Kefi K, Braud L, Schmitt A, Yacia A, Schirmann A, Hersant B, Sid-Ahmed M, Ben Larbi S, Komrskova K, Rohlena J, Relaix F, Neuzil J, Rodriguez AM (2021) Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab 33(3):688–690. https://doi.org/10.1016/j.cmet.2021.02.003
Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F, Chang H, Zhao G, Gao W, Li Y, Wang Q (2020) Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice. Clin Sci 134(16):2161–2175. https://doi.org/10.1042/cs20200530
Shi C, Guo H, Liu X (2021) Platelet mitochondria transplantation rescues hypoxia/reoxygenation-induced mitochondrial dysfunction and neuronal cell death involving the FUNDC2/PIP3/Akt/FOXO3a axis. Cell Transplant 30:9636897211024210. https://doi.org/10.1177/09636897211024210
Hayashi T, Tanaka S, Hori Y, Hirayama F, Sato EF, Inoue M (2011) Role of mitochondria in the maintenance of platelet function during in vitro storage. Transfus Med 21(3):166–174. https://doi.org/10.1111/j.1365-3148.2010.01065.x
Bynum JA, Meledeo MA, Getz TM, Rodriguez AC, Aden JK, Cap AP, Pidcoke HF (2016) Bioenergetic profiling of platelet mitochondria during storage: 4°C storage extends platelet mitochondrial function and viability. Transfusion 56(Suppl 1):S76-84. https://doi.org/10.1111/trf.13337
Zhao J, Xu B, Chen G, Zhang Y, Wang Q, Zhao L, Zhou H (2019) Cryopreserved platelets augment the inflammatory response: role of phosphatidylserine- and P-selectin-mediated platelet phagocytosis in macrophages. Transfusion 59(5):1799–1808. https://doi.org/10.1111/trf.15183
Acknowledgements
The authors would like to thank all blood volunteers for their participation in this study. We thank the Electron Microscopy Center of Fourth Military Medical University for this study.
Funding
The present work was supported by the National Natural Science Foundation of China (No. 82170226).
Author information
Authors and Affiliations
Contributions
ED, YTC, and WTW performed the experiments and analyzed and interpreted the data. YTC and LLZ drafted the manuscript. NA and WY contributed reagents. YZC and JY conceived and designed the experiments. YZC and YTC revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All authors’ consent for the use of biospecimens was obtained in accordance with the Declaration of Helsinki. The experimental procedures were performed according to the requirements of the Ethics Committee of Xijing Hospital.
Consent to participate
Informed consent was obtained from all blood volunteers for being included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
E. Dang, Yutong Chen, and Wenting Wang contributed equally as the first authors. Yaozhen Chen and Jing Yi contributed equally as the senior authors.
Supplementary information
Below is the link to the electronic supplementary material.
277_2023_5580_MOESM1_ESM.tif
Supplementary file1 (TIF 8288 KB) Detection of the count of platelets in PRP under different storage temperature and storge time. (A) A schematic diagram of PRP treatment. (B) The count of platelets in PRP on 0 day, 14th day, 28th day at -30℃ (N = 5). (C) The count of platelets in PRP on 0 day, 14th day, 28th day at -80℃ (N = 5). (D) The count of platelets in PRP at -30℃ and -80℃ on 14th day (N = 5). (E) The count of platelets in PRP at -30℃ and -80℃ on 28th day (N = 5). ns, no statistical significance. PLT: platelets in PRP; 0d: platelets in fresh PRP, as control group; 14d: platelets in PRP were stored on the 14th day; 28d: platelets in PRP were stored on the 28th day; -30℃: platelets in PRP stored at -30℃; -80℃: platelets in PRP stored at -80℃.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dang, E., Chen, Y., Wang, W. et al. A comparative study of platelet storage lesion in platelet-rich plasma under cryopreservation. Ann Hematol 103, 631–643 (2024). https://doi.org/10.1007/s00277-023-05580-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05580-0