Abstract
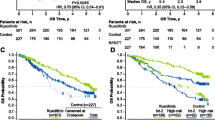
In the MYF2001 trial, treatment of Janus kinase (JAK) inhibitor-relapsed/refractory intermediate-2 or high-risk myelofibrosis (MF) with imetelstat 9.4 mg/kg every 3 weeks demonstrated encouraging median overall survival of 29.9 months. To provide historical context, external real-world data (RWD) were collected from a study of 96 patients who had discontinued ruxolitinib and were subsequently treated with best available therapy (BAT) at Moffitt Cancer Center. A closely matched cohort was identified using the MYF2001 eligibility criteria, including patients with MF who had discontinued ruxolitinib due to lack or loss of response. Overall survival was measured from time of JAK inhibitor discontinuation to death or censored at last follow-up. To improve comparability, propensity score weighting approaches using average treatment effect for overlap population (ATO) and stabilized inverse probability treatment weighting (sIPTW) were used for 10 critical baseline covariates. Fifty-seven patients treated with imetelstat 9.4 mg/kg from MYF2001 and 38 patients treated with BAT from RWD were analyzed with improved balanced baseline covariates after propensity score adjustment, showing significantly lower risk of death with imetelstat compared with BAT (hazard ratio: 0.35; p = 0.0019). With sIPTW, results were similar. Results of sensitivity analyses were consistent with the primary analysis. In conclusion, treatment with imetelstat was associated with longer overall survival compared to BAT (30 vs 12 months, respectively) in closely matched patients with MF after JAK inhibitor failure, warranting further evaluation of imetelstat in this poor-prognosis patient population.



Similar content being viewed by others
Availability of data and material
Deidentified clinical trial data that underlie the reported results can be requested by any qualified researchers who engage in rigorous, independent scientific research. Proposals for access must include a research proposal for Geron’s review and a Statistical Analysis Plan, and if approved, access will require the execution of a Data Sharing Agreement. Data requests can be submitted starting 3 months after publication for a period of 12 months after publication, with possible extensions considered. For more information on the process, or to submit a request, please contact info@geron.com.
References
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, Guglielmelli P, Pungolino E, Caramella M, Maffioli M, Pascutto C, Lazzarino M, Cazzola M, Tefferi A (2010) A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 115(9):1703–1708
Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, Van Dyke D, Hanson C, Wu W, Pardanani A, Cervantes F, Passamonti F, Tefferi A (2011) DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 29(4):392–397
Vannucchi AM (2017) Guglielmelli P (2017) What are the current treatment approaches for patients with polycythemia vera and essential thrombocytopenia? Hematology Am Soc Hematol Educ Program 1:480–488
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366(9):787–798
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH Jr, Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM (2012) A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366(9):799–807
Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, Masszi T, Mishchenko E, Jourdan E, Vannucchi AM, Drummond MW, Jurgutis M, Kuliczkowski K, Gheorghita E, Passamonti F, Neumann F, Patki A, Gao G, Tefferi A (2015) Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 1(5):643–651
Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Tiu RV, Zachee P, Jourdan E, Winton E, Silver RT, Schouten HC, Passamonti F, Zweegman S, Talpaz M, Lager J, Shun Z, Mesa RA (2017) Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomized, phase 2, multicenter study. Lancet Haematol 4(7):e317–e324
Abdelrahman RA, Begna KH, Al-Kali A, Hogan WJ, Litzow MR, Tefferi A (2015) Revised assessment of response and long-term discontinuation rates among 111 patients with myelofibrosis treated with momelotinib or ruxolitinib. Leukemia 29(2):498–500
Kuykendall AT, Shah S, Talati C, Al Ali N, Sweet K, Padron E, Sallman DA, Lancet JE, List AF, Zuckerman KS, Komrokji RS (2018) Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann Hematol 97(3):435–441
Mascarenhas J, Mehra M, He J, Potluri R, Loefgren C (2020) Patient characteristics and outcomes after ruxolitinib discontinuation in patients with myelofibrosis. J Med Econ 23(7):721–727
McNamara C, Spiegel J, Xu W, Kennedy J, Arruda A, Yu M, Claudio J, Siddiq N, Cheung V, Malik S, Tierens A, Maze D, Sibai H, Gupta V (2019) Long-term follow-up of JAK inhibitor (JAKI) treated patients with myelofibrosis: impact of molecular enhanced integrated scoring systems and type of JAKI therapy failure [abstract]. HemaSphere 3(S1):672–673
Newberry KJ, Patel K, Masarova L, Luthra R, Manshouri T, Jabbour E, Bose P, Daver N, Cortes J, Kantarjian H, Verstovsek, (2017) Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood 130(9):1125–1131
Palandri F, Palumbo GA, Bonifacio M, Breccia M, Latagliata R, Martino B, Polverelli N, Abruzzese E, Tiribelli M, Nicolosi M, Bergamaschi M, Tieghi A, Iurlo A, Sgherza N, Cavazzini F, Isidori A, Binotto G, Ibatici A, Crugnola M, Heidel F, Bosi C, Bartoletti D, Auteri G, Catani L, Cuneo A, Aversa F, Semenzato G, Cavo M, Vianelli N, Benevolo G (2018) Durability of spleen response affects the outcome of ruxolitinib-treated patients with myelofibrosis: results from a multicentre study on 284 patients. Leuk Res 74:86–88
Schain F, Vago E, Song C, He J, Liwing J, Löfgren C, Björkholm M (2019) Survival outcomes in myelofibrosis patients treated with ruxolitinib: a population-based cohort study in Sweden and Norway. Eur J Haematol 103(6):614–619
Mascarenhas J, Komrokji RS, Palandri F, Martino B, Niederwieser D, Reiter A, Scott BL, Baer MR, Hoffman R, Odenike O, Vannucchi AM, Bussolari J, Zhu E, Rose E, Sherman L, Dougherty S, Sun L, Huang F, Wan Y, Feller FM, Rizo A, Kiladjian JJ (2021) Randomized, single-blind, multicenter phase II study of two doses of imetelstat in relapsed or refractory myelofibrosis. J Clin Oncol doi: https://doi.org/10.1200/JCO.20.02864. Epub ahead of print.
Cole SR, Hernán MA (2004) Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 75(1):45–49
Xie J, Liu C (2005) Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 24(20):3089–3110
Li F, Morgan KL, Zaslavsky AM (2018) Balancing covariates via propensity score weighting. J Am Stat Assoc 113(521):390–400
Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, Szoke A, Drummond M, Pristupa A, Granston T, Daly R, Al-Fayoumi S, Callahan JA, Singer JW, Gotlib J, Jamieson C, Harrison C, Mesa R, Verstovsek S (2018) Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol 4(5):652–659
Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D, Passamonti F, Winton EF, Dong H, Kawashima J, Maltzman JD, Kiladjian JJ, Verstovsek S (2018) Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY-2): a randomised, open-label, phase 3 trial. Lancet Haematol 5(2):e73–e81
Celgene Corporation. Inrebic® (fedratinib) prescribing information. Revised 08/2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf. Accessed on 11 November 2020.
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46(3):399–424
European Medicines Agency. ICH E10: Choice of Control Groups and Related Issues in Clinical Trials. International Conference on Harmonization of Technical Requirements for Regulation of Pharmaceuticals for Human Use. January 2001. Available at https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-10-choice-control-group-clinical-trials-step-5_en.pdf. Accessed on 17 August 2021.
Mascarenhas J, Harrison C, Kiladjian J-J, Mesa R, Komrokji RS, Koschmieder S, Vannucchi A, Berry T, Sherman L, Dougherty S, Sun L, Huang F, Wan Y, Feller F, Rizo A, Verstovsek S (2020) A randomized open-label, phase 3 study to evaluate imetelstat versus best available therapy (BAT) in patients with intermediate-2 or high-risk myelofibrosis (MF) refractory to Janus kinase (JAK) inhibitor [abstract]. Blood 136(S1):2194
Acknowledgements
Editorial support for this publication was provided by Laurie Orloski, of InSeption Group, funded by Geron Corporation.
Funding
This work was supported by research funding from Janssen Research & Development LLC and Geron Corporation.
Author information
Authors and Affiliations
Contributions
A.T.K, R.S.K, Y.W., J.B., and L.S. designed the study. Y.W., L.S., Q.X., E.Z., and J.B. made the figures; all authors collaborated in writing and reviewing the paper and approved the final submitted version.
Corresponding author
Ethics declarations
Ethics approval
An independent ethics committee/institutional review board (IRB) had given full approval of the protocol, any amendments, and the informed consent forms for MYF2001. IRB approval was obtained for the collection, analysis, and sharing of real-world data.
Consent to participate
Written informed consent was received by each patient (or a legally acceptable representative) before performance of any study-related activity in MYF2001. Patients in the real-world cohort were not consented for this analysis, as the data were derived per retrospective chart review.
Conflict of interest
A.T.K.—research support: Prelude, Sierra Oncology, Protagonist; Speakers Bureau: Novartis, Blueprint; advisory boards/honoraria: Celgene/BMS, Abbvie, Pharmaessentia, Novartis, Blueprint, CTI Biopharma, Incyte; J.M.—research support: Incyte, Janssen, CTI Bio, PharmaEssentia, Novartis, Roche, Kartos, Promedior Merck; consultancy: Promedior, Prelude, Galecto, Incyte, Celgene, Kartos, Geron; J.-J.K.—advisory board: Novartis, AOP Orphan, BMS, AbbVie; A.M.V.—advisory board: Novartis, Abbvie, Celgene, Incyte, Blueprint; Fees (lectures): Novartis, Celgene, Takeda, CTI; Y.W., F.F., L.S., A.R.—employment/equity ownership: Geron Corporation; J.W., Q.X., E.Z., J.B.—employment/equity ownership: Janssen Research and Development; R.S.K.—Speakers Bureau: Celgene/BMS, JAZZ, Agios, Abbvie; advisory boards/honoraria: Celgene/BMS, JAZZ, Agios, Abbvie, Novartis.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuykendall, A.T., Sun, L., Mascarenhas, J. et al. Favorable overall survival with imetelstat in relapsed/refractory myelofibrosis patients compared with real-world data. Ann Hematol 101, 139–146 (2022). https://doi.org/10.1007/s00277-021-04683-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04683-w