Abstract
The role of allogeneic hematopoietic stem cell transplantation (allo-SCT) in multiple myeloma is controversial. We analyzed the results of 205 patients transplanted in one center during 2000–2017. Transplantation was performed on 75 patients without a previous autologous SCT (upfront-allo), on 74 as tandem transplant (auto-allo), and on 56 patients after relapse. Median overall survival (OS) was 9.9 years for upfront-allo, 11.2 years for auto-allo, and 3.9 years for the relapse group (p = 0.015). Progression-free survival (PFS) was 2.4, 2.4, and 0.9 years, respectively (p < 0.001). Non-relapse mortality at 5 years was 8% overall, with no significant difference between the groups. Post-relapse survival was 4.1 years for upfront-allo and auto-allo, and 2.6 years for the relapse group (p = 0.066). Survival of high-risk patients was reduced. In multivariate analysis, the auto-allo group had improved OS and chronic graft-versus-host disease was advantageous in terms of PFS, OS, and relapse incidence. Late relapses occurred in all groups. Allo-SCT resulted in long-term survival in a small subgroup of patients. Our results indicate that auto-allo-SCT is feasible and could be considered for younger patients in the upfront setting.
Similar content being viewed by others
Introduction
Allogeneic stem cell transplantation (allo-SCT) is thus far the only potentially curative treatment approach in multiple myeloma (MM), but only a fraction of patients are eligible for it. The use of allo-SCT is limited by transplant-related mortality (TRM) that can rise to 41% with myeloablative conditioning (MAC) [1]. With reduced-intensity conditioning (RIC), the TRM is lower (10–15%), but the risk of relapse is higher than that with MAC [2,3,4]. Reduced toxicity conditioning may offer survival outcomes equal to that of MAC but a more acceptable TRM [5, 6].
The role of allo-SCT in the treatment of MM is under debate. This is based on the contradicting results of previous studies over the survival advantage allo-SCT may offer over autologous stem cell transplantation (ASCT), and the disappointing relapse rate after allo-SCT [2,3,4, 7,8,9]. However, a proportion of patients seem to remain in long-term remission [3, 6, 8]. It is also unclear, whether the graft-versus-myeloma effect of allo-SCT [10] can overcome the poor prognosis of high-risk (HR) patients [11,12,13,14,15,16].
Allo-SCT can be considered in MM as the first-line treatment, with or without a previous ASCT, or in relapsed disease as a salvage treatment. Current guidelines recommend considering allo-SCT only after disease relapse or as a part of a clinical trial [17,18,19]. Still, allo-SCTs are performed outside of clinical trials in considerable amounts [20].
In this retrospective single-center study, we report the results of allo-SCT performed in three different settings: upfront without a previous ASCT, after ASCT as a tandem transplant (auto-allo), and after relapse. Our aim was to determine the outcomes of these transplant strategies. Our second point of interest was the outcome of patients with HR cytogenetics.
Methods
Study design and population
We included all patients with MM who underwent allo-SCT between January 2000 and December 2017 in Helsinki University Hospital Stem Cell Transplantation Unit. Primary plasma cell leukemia was an exclusion criterion. The data was collected from the European Group for Blood and Marrow Transplantation (EBMT) database. All patients provided written informed consent for the EBMT reporting. Data not included in the EBMT reports was collected from the medical records as approved by our institutional review board. This study was conducted according to the Declaration of Helsinki, International Conference of Harmonization and Guidelines for Good Clinical Practice.
Helsinki University Hospital is one of the two Finnish allogeneic stem cell transplantation centers. Patients are referred to us from all parts of Finland except for the southwest district. The standard operating procedures of our transplantation unit have recommended allo-SCT to be considered in an upfront setting for young patients, mostly under 50–55 years of age with extramedullary disease or advanced bone disease. The choice of transplanting the patients with or without a previous ASCT was based on clinical decision-making.
Definitions and endpoints
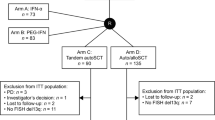
For the purpose of this analysis, patients were divided into three subgroups, referred to here as “therapy groups”: (1) “upfront-allo” group who received allo-SCT in first line without a previous ASCT, (2) “auto-allo” group, where allo-SCT was performed as a preplanned tandem therapy after ASCT, and (3) “relapse” group who received allo-SCT after at least one relapse, with or without a previous ASCT.
HR cytogenetics was defined as the presence of del17p, t(4;14), or t(14;16) [21]. In 2000–2002, chromosome analysis was performed with G band karyotyping. Since 2003, the use of fluorescence in situ hybridization started to increase, with a widening array of probes. In 2010, the incorporation of CD138+ plasma cell selection method further improved the sensitivity of analyses. Therefore, data of HR cytogenetics was available to us sporadically in 2003–2009 and systematically from the year 2010 on.
Conditioning regimen intensities and graft-versus-host disease (GVHD) were graded according to previously published criteria [22,23,24,25]. The International Myeloma Working Group (IMWG) criteria were used to define MM disease status and relapse [26].
The data cutoff was 20 December 2019. The primary endpoint was overall survival (OS) according to the therapy group. Secondary endpoints included progression-free survival (PFS), relapse incidence (RI), post-relapse survival (PRS), non-relapse mortality (NRM), and GVHD- and relapse-free survival (GRFS). GRFS was defined as being alive with neither grade 3-4 acute GVHD (aGVHD), systemic therapy-requiring chronic GVHD (cGVHD), nor disease relapse at any time point [27].
Statistical analysis
Statistical analyses were performed according to the EBMT Statistical Guidelines [28]. Patient-, disease-, and transplant-related variables of the therapy groups were compared using Pearson’s chi-square test or Fisher’s exact test for categorical variables, and Mann-Whitney test or Kruskal-Wallis test for continuous variables. The probabilities of OS, PFS, PRS, and GRFS were calculated using the Kaplan-Meier method and the log-rank test for univariate comparisons. Acute and chronic GVHD, RI, and NRM were calculated by using the cumulative incidence (CI) estimator to accommodate competing risks. Gray’s test was used for between-group tests. For NRM, relapse was the competing risk, and for RI, the competing risk was death without relapse. Multivariate analyses for OS and PFS were performed using Cox proportional hazards regression model and for RI by the Fine-Gray method. Gender, age, and Karnofsky performance status of the patient, International Staging System (ISS) stage, presence of extramedullary disease, number of treatment lines and disease status before allo-SCT, therapy group, year of the allo-SCT, conditioning regimen intensity, graft type, donor source, donor/recipient gender mismatch and CMV status, and presence of GVHD were tested in univariate analyses. Variables with p-values < 0.3 were taken into multivariate analyses. p-values are two-sided. Statistical analyses were performed with the SPSS 25 (SPSS inc./IBM, Armonk, NY, USA) and R version 3.6.2. (R Core Team. 2019) [29] software packages.
Results
Patient and transplant characteristics
Baseline characteristics are summarized in Table 1. Eighteen patients (9%) participated in a clinical study; 15 in the EBMT-NMAM2000 study [4] and three in a treosulfan-based conditioning regimen study [30].
Gender, MM subtype, ISS stage, and HR cytogenetics were balanced across the groups. The patients in the upfront-allo group were younger, had more extramedullary disease, and received bone marrow grafts more often than patients in the other two groups. The patients in the relapse group were older than those in the other groups. Eighty-nine (43%) transplantations were performed in 2000–2007 and 116 (57%) in 2008–2017. The most common induction therapy was doxorubicin and dexamethasone, with or without vincristine (AD/VAD), mostly given in 2000–2006. Thalidomide-based combinations were used in first line in 2006–2013, bortezomib and dexamethasone (BorDex) from 2007 onwards, and bortezomib in combination with cyclophosphamide and dexamethasone (VCD) or lenalidomide and dexamethasone (RVD) from 2012 onwards. The majority of the patients had received treatment with immunomodulators or proteasome inhibitors before the allo-SCT, with 62% of the patients being treated with bortezomib and 28% with thalidomide. Lenalidomide was reimbursed in Finland in 2010 and 28% of the patients had received it before the transplantation. Four different conditioning regimens were used. Maintenance treatment was not routinely used after allo-SCT.
The median number of CD34+ cells in peripheral blood grafts was 6.1 (range, 1.6–15.3) × 106/kg. In bone marrow grafts, the median total nucleated cell count of 25 grafts was 2.8 (range, 1.6–5.6) × 108/kg, and the median number of mononuclear cells of 14 grafts 0.5 (range, 0.2–1.4) × 108/kg.
Response and GVHD
Overall response rate (ORR) was 86%, with 51% of the patients achieving sCR or CR. Out of 164 patients with available data on chimerism, 157 (96%) achieved full donor chimerism. Cumulative incidence of aGVHD grades 2–4 at day 100 was 24%. Chronic GVHD occurred in 62% of the patients and it was extensive in 47% (Table 2).
Multivariate analysis for factors affecting survival and relapse incidence
Table 3 shows the results of the multivariate analysis. Independent factors for longer OS were female gender, lower ISS stage, MAC, auto-allo therapy group, and cGVHD. Acute GVHD grade ≥ 2 reduced OS. Predictive factors for reduced PFS were higher ISS stage, relapse group, and aGVHD grade ≥ 2. Chronic GVHD predicted longer PFS. Chronic GVHD, age ≤ 55 years, Karnofsky performance scale ≥ 80, lower number of prior treatment lines, and not having extramedullary disease were associated with lower RI.
To further examine the effect of cGVHD on survival, a landmark analysis was done with patients (n = 199) who had survived at least for 100 days after the transplantation. The OS difference remained significant. In this analysis, median OS was 11.4 years (95% CI, 6.9–15.8) with and 3.7 years (95% CI, 2.2–5.1) without cGVHD (p < 0.001).
Survival outcomes by therapy groups
With a median follow-up of 4.3 years (range, 0–18.4) for the entire cohort and 5.2 years (range 1.1–18.4) for patients surviving over 1 year, the median OS was 7.4 years (95% CI: 5.1–9.8) (Fig. 1a). By therapy groups, the median OS were 9.9 years (95% CI: 6.0–13.9), 11.2 years (95% CI: 3.8–18.7), and 3.9 years (95% CI: 3.0–4.9) (p = 0.015) for upfront-allo, auto-allo, and relapse groups, respectively (Fig. 2a).
The median PFS of the entire cohort was 1.8 years (95% CI: 1.3–2.3) with a median follow-up of 1.6 years (range, 0–17.2) (Fig. 1b). By therapy groups, the median PFS were 2.4 years (95% CI: 1.1–3.8), 2.4 years (95% CI: 1.6–3.1), and 0.9 years (95% CI: 0.5–1.4) (p < 0.001) for upfront-allo, auto-allo, and relapse groups, respectively (Fig. 2b). The median OS and PFS of the eighteen patients participating in the clinical studies did not differ from other patients in our study.
When the disease status in the auto-allo group before allo-SCT was sCR or CR (n = 22), median OS was not reached, compared with median OS of 11.2 years (95% CI, 4.5–18.0) with VGPR or PR (n = 49), and 6.1 years (95% CI not calculated, n = 2) with disease status less than PR (p = 0.627). This effect was not seen in the other two therapy groups. Disease status before first ASCT did not affect survival outcomes in auto-allo or relapse groups. The number of previous treatment lines did not significantly affect OS in any therapy group. In the relapse group, median OS were 3.9 years (95% CI, 1.9–5.8) after two, 6.7 years (95% CI, 2.3–11.1) after three, and 2.7 years (95% CI, 0–6.4) after four or more previous lines (p = 0.363). The median PFS in the relapse group were 0.8 years (95% CI, 0–1.5) after two, 1.3 years (95% CI, 0.3–2.3) after three, and 0.6 years (95% CI, 0–1.3) after four or more previous lines (p = 0.482). There was no difference in OS in any therapy group according to whether the patients had received treatment with immunomodulators or proteasome inhibitors as induction therapy or in later line before the allo-SCT or not.
Patients with cGVHD had median OS of 11.7 years (95% CI, 7.2–16.1) compared with 3.6 years (95% CI, 2.3–5.0) (p < 0.001) for those without it. Median PFS was 2.6 years (95% CI, 1.9–3.3) for patients with and 1.0 years (95% CI, 0.5–1.5) (p < 0.001) for patients without cGVHD. Median GRFS was 0.6 years (95% CI, 0.5–0.7) for the entire cohort, with 18% at 2 years and 10% at 5 years. The cumulative incidence of NRM was 8% at 5 years overall (Fig. 1c) and it was the highest (12%) in the auto-allo group (Fig. 2c).
Relapse incidence and post-relapse survival by therapy groups
Relapse incidence for the entire cohort was 62% at five and 68% at ten years (Fig. 1c). By therapy groups, RI at 5 years were 56% for upfront-allo, 51% for auto-allo, and 82% for relapse (p < 0.001) (Fig. 2c).
The median PRS for all patients was 3.5 years (95% CI, 2.7–4.2); 68% at 2, 38% at 5, and 25% at 10 years. For upfront-allo, auto-allo, and relapse groups, the median PRS were 4.1 years (95% CI, 0.5–8.1), 4.1 years (95% CI, 1.9–6.3), and 2.6 years (95% CI, 2.0–3.2), respectively (p = 0.066). In patients with cGVHD, the median PRS was significantly longer with 6.5 years (95% CI, 3.2–9.9), compared with 2.1 years (95% CI, 1.1–3.0) in others (p < 0.001).
Forty-nine patients were treated with donor lymphocyte infusion (DLI), nine of them prophylactically. When comparing patients who received DLI for treatment of PD with others, there was no difference in PRS (data not shown).
Patients with high-risk disease
A complete cytogenetic analysis was performed in 87 (42%) patients and 31 (15%) had HR features. Their median OS was reduced compared with standard risk (SR) patients (Table 4). There was no difference in NRM between patients with HR and SR cytogenetics. Median PRS was 1.1 years (95 % CI, 0.5–1.7) for patients with HR cytogenetics (n = 20) and 4.1 years (95 % CI, 2.1–6.0) for SR cytogenetics (n = 36) (p = 0.002). The information on the ISS stage was available for 175, the R-ISS stage for 82, and IMWG risk stratification for 86 patients; and all correlated with survival outcomes (Table 4).
Outcomes by conditioning regimens
Patients transplanted with MAC had longer median OS of 10.9 years (95% CI, 7.0–14.9) compared with 5.2 years (95% CI, 2.5–7.9) in patients receiving transplant after RIC (p = 0.027). There was no difference in PFS, RI, or NRM between MAC and RIC.
When comparing the four different conditioning regimens used (Table 1), the median OS was 11.7 years (95% CI, 6.3–17.0), 9.9 years (95% CI, 5.9–14.0), 6.4 years (95% CI, 2.1–10.7), and 4.1 years (95% CI, 1.0–7.2) (p = 0.042); and the median PFS 3.4 years (95% CI, 1.2–5.5), 2.2 years (95% CI, 1.7–2.7), 1.6 years (95% CI, 0.7–2.4), and 1.2 years (95% CI, 0.7–1.7) (p = 0.009) after conditioning with CyTBI, Treo14, FluTBI, and Treo-RIC, respectively. PFS at 5 years were 43% for CyTBI, 18% for Treo14, 35% for FluTBI, and 19% for Treo-RIC conditioning (p = 0.025). NRM was highest for FluTBI regimen with 17% at 5 years, whereas it was 7% with CyTBI, 3% with Treo14, and 5% with Treo-RIC (p = 0.041).
Patients with long progression-free survival
There were 43 patients who were progression-free at 5 years after allo-SCT. Their median OS was not reached and median PFS was 15.2 years (95% CI, 9.0–21.4). The characteristics of these long-term survivors are summarized in Table 5. Twenty-six (60%) of them were transplanted before 2008 and the induction therapy was AD or VAD in 23 (54%) and a thalidomide-based combination in 10 (23%) patients. Six (14%) received a bortezomib-based induction. The number of pre-allo-SCT treatment lines was 1–2 in 86%. Only 16% had ISS stage III disease and 26% extramedullary disease. All but four of these long-term survivors were transplanted early in the course of the disease. The majority had reached a good response before allo-SCT, with 18% sCR/CR and 77% VGPR/PR. The majority (72%) had chronic GVHD.
Discussion
We have conducted allo-SCT for over 200 patients with MM, mostly (73%) as first-line therapy (upfront-allo and auto-allo). Of the three therapy groups, the auto-allo group had the longest median OS, 11.2 years. Other studies with 6- to 7-year follow-up times after auto-allo SCT have resulted in a median OS of 5.9–11.4 years [11, 15, 31].
We had 75 patients who received allo-SCT without a prior ASCT or relapse. The median OS of this group was nearly 10 years. Previous studies have reported median OS time of 1.5–3.3 years with first-line allo-SCT [1, 20]. The 5-year NRM in our upfront-allo-SCT group, with CyTBI conditioning given to 53 patients, was significantly lower than the 30–41% NRM reported in the literature [1, 20]. In fact, NRM was low overall in our study and compares well to the NRM of 10–37% reported elsewhere [5, 6, 15, 31, 32].
Compared with the OS, PFS was considerably shorter in both upfront-allo and auto-allo groups of our study. This is in line with the 0.8–4.0 years reported previously [1, 6, 11, 15, 20, 33]. The discrepancy between a long median OS but disappointingly short PFS for upfront-allo-SCT is explained by the long PRS of 4.1 years that is probably largely due to modern and more efficacious drug combinations used to treat relapse. Costa et al. performed a pooled analysis with 1338 newly diagnosed MM patients from four prospective auto-allo trials, with a median PRS of 5.2 years [34]. Others have reported PRS of 1.8–6.4 years after allo-SCT [15, 33, 35, 36]. In our study, the RI curve seemed to form a plateau at 10–15 years, indicating that 20–25% of the patients achieve long-term remission, as seen in previous studies with a long follow-up [3, 6, 8]. These findings are perhaps an indication of the importance of immunology and effective graft-versus-myeloma effect [32, 37, 38]. Also, a previous upfront allo-SCT does not seem to worsen the response to relapse treatment.
In regard to allo-SCT for relapsed/refractory MM, the outcomes were clearly inferior to the other two therapy groups. Patients relapsed early despite only four patients (7%) having PD at the time of allo-SCT. Even sCR/CR at the time of allo-SCT did not result in improved survival. The patients in the relapse group were not very heavily pretreated as 76% had received only 2–3 previous treatment lines before allo-SCT. The PRS of the relapse group was only 2.6 years. In the study by Greil et al. with retrospective analysis of 109 patients, those transplanted first line had better OS and PFS than those receiving allo-SCT in relapsed/refractory phase [6].
Our observation of aGVHD incidence of 24%, the detrimental effect of aGVHD, and the beneficial effect of cGVHD on the outcome of the patients is in line with several previous studies [12, 32, 39]. For cGVHD, the rate of 62% in our study was in the upper range compared with 27–67% reported elsewhere [16, 31, 39, 40]. This translated to the GRFS median of less than a year. However, GRFS does not evaluate cGVHD in a time-dependent manner and there may also be differences between the centers in the threshold for initiating the systemic cGVHD treatment. It should be noted that cGVHD may affect quality of life of the patients, but this data was not available to us.
We were able to define cytogenetic risk according to current criteria [21] for less than half of the patients, approximately one-third of them with HR cytogenetics. Several studies have shown that allo-SCT could overcome the adverse prognosis of high cytogenetic risk [6, 11,12,13,14], while others have not [15, 16]. In our study, allo-SCT did not seem to benefit patients with HR cytogenetics despite a majority of them receiving allo-SCT in an upfront setting. Their overall and post-relapse survival was markedly reduced compared with SR patients. The immunological effect of allo-SCT may be too slow to revert the rapid disease progression in patients with HR cytogenetics. However, our results should be interpreted with caution due to the limited number of patients.
In our study, MAC associated with a better OS than RIC, but there was no difference in PFS or RI. There were more allo-SCTs performed in relapsed/refractory MM in RIC than in the MAC group, but nevertheless, in multivariate analysis, MAC retained its independent prognostic value. In a large retrospective EBMT analysis, MAC was associated with poor OS in 1991–2002 but not after that [41]. In our study, the year of transplantation did not affect the survival outcomes.
Treosulfan-based conditioning has led to favorable responses and low NRM in some studies [5, 42], thus characterized as reduced-toxicity conditioning. In our study, 98 patients received treosulfan-based conditioning, 33 treo14, and 65 treo-RIC. Both resulted in good 5-year NRM rates of 3% and 5% but median PFS was equal to other conditioning regimens.
The serological status of myeloma before allo-SCT did not affect the survival outcomes in our study, although the proportion of patients in PD before allo-SCT was small. Even if limited by a small number of patients, it was interesting to see that in the auto-allo group, patients with sCR/CR before allo-SCT (n = 22) had a very long median PFS of 7.5 years (data not shown). This tendency did not show in upfront-allo group. This may be an indicator of the debulking effect of ASCT resulting in deeper response than anti-MM drugs alone.
Our study has some obvious weaknesses, including its retrospective nature and the long time span of 17 years. There has been marked development during these years in cytogenetic analyses, anti-MM drugs, and supportive care after allo-SCT. This evolution is certainly reflected in the changes in the guidelines regarding indications for allo-SCT in myeloma [17,18,19, 43]. Even if there did not seem to be great differences between the patients in the three therapy groups, we cannot exclude the possibility that the allocation criteria for one or another transplant strategy could have had an effect on the results. Especially during the first half of our study period, the national reimbursement policy may have affected patient referral strategy. Also, data on induction therapy response, and different post-relapse therapies, was not available to us except in the case of a DLI. As the purpose of our study was to analyze the outcome of different allo-SCT timings, we did not have an ASCT group for comparison. The referral area and post-SCT follow-up strategy of ASCT patients is different from allo-SCT patients in our center. Therefore, we would also not have been able to report the outcomes of ASCT patients with accuracy similar to allo-SCT.
In addition to allo-SCT, there are other treatment approaches with a possible chance for cure in MM, as the donor-derived chimeric antigen receptor T cell (CAR-T) or CAR-NK-cell therapy [44, 45]. These therapy forms may even replace allo-SCT in the future. A tempting approach could also be combining immunological treatment approaches [46] to allo-SCT. Adding novel agents to the conditioning regimen has shown promising results [47]. Maintenance treatment with novel agents after allo-SCT has also been studied [48,49,50]. Maintenance therapy should possibly be guided by measurable residual disease and donor chimerism. However, in the work by Rasche et al. [13] and Chhabra et al. [35], the majority of patients with relapse still displayed full donor chimerism in blood or bone marrow.
As a conclusion, in this single-center study with a fairly large number of patients and 5-year follow-up time, we observed relatively good outcomes in terms of long OS and low NRM in upfront-allo and auto-allo transplantations. Auto-allo-SCT as an upfront treatment seemed to be the best approach of these regimes in our material. However, achieving a long-lasting remission was challenging as PFS was relatively short. Patients with long PFS of at least 5 years were characterized by allo-SCT performed early in the course of the disease, ISS stage I and chronic GVHD. PRS was quite good, possibly due to graft-versus-myeloma effect as indicated by the beneficial effect of cGVHD and modern MM treatment. Patients with HR cytogenetics did not seem to benefit from allo-SCT. Further studies are needed to show if the relapse rate could be decreased by combining immunomodulatory drugs either to the conditioning regimen or to the post-allo-SCT period.
Data availability
The datasets generated and analyzed during the study are available from the corresponding author on reasonable request.
References
Björkstrand B, Ljungman P, Svensson H, Hermans J, Alegre A, Apperley J et al (1996) Allogeneic stem cell transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood 88:4711–4718. https://doi.org/10.1182/blood.V88.12.4711.bloodjournal88124711
Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, Lamy T, Marit G, Maloisel F, Berthou C, Dib M, Caillot D, dePrijck B, Ketterer N, Harousseau JL, Sotto JJ, Moreau P, for the Intergroupe Francophone du Myélome and the Swiss Group for Clinical Cancer Research (2006) Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood 107:3474–3480. https://doi.org/10.1182/blood-2005-09-3869
Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, Giaccone L, Sorasio R, Omedè P, Baldi I, Bringhen S, Massaia M, Aglietta M, Levis A, Gallamini A, Fanin R, Palumbo A, Storb R, Ciccone G, Boccadoro M (2007) A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med 356:1110–1120. https://doi.org/10.1056/NEJMoa065464
Gahrton G, Iacobelli S, Björkstrand B, Hegenbart U, Gruber A, Greinix H, Volin L, Narni F, Carella AM, Beksac M, Bosi A, Milone G, Corradini P, Schönland S, Friberg K, van Biezen A, Goldschmidt H, de Witte T, Morris C, Niederwieser D, Garderet L, Kröger N, EBMT Chronic Malignancies Working Party Plasma Cell Disorders Subcommittee (2013) Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long term results of the EBMT-NMAM2000 study. Blood 121:5055–5063. https://doi.org/10.1182/blood-2012-11-469452
Gran C, Wang J, Nahi H, Koster L, Gahrton G, Einsele H, Niittyvoupio R, Edinger M, Beelen D, Ciceri F, Bornhäuser M, Finke J, Wreede LC, Ljungman P, Mielke S, Tischer J, Garderet L, Schönland S, Yakoub-Agha I, Kröger N (2020) Treosulfan conditioning for allogeneic transplantation in multiple myeloma - improved overall survival in first line haematopoietic stem cell transplantation - a large retrospective study by the Chronic Malignancies Working Party of the EBMT. Br J Haematol 189:e213–e217. https://doi.org/10.1111/bjh.16642
Greil C, Engelhardt M, Ihorst G, Schoeller K, Bertz H, Marks R, Zeiser R, Duyster J, Einsele H, Finke J, Wäsch R (2019) Allogeneic transplantation of multiple myeloma patients may allow long-term survival in carefully selected patients with acceptable toxicity and preserved quality of life. Haematologica 104:370–379. https://doi.org/10.3324/haematol.2018.200881
Björkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, Narni F, Musto P, Beksac M, Bosi A, Milone G, Corradini P, Goldschmidt H, de Witte T, Morris C, Niederwieser D, Gahrton G (2011) Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol 29:3016–3022. https://doi.org/10.1200/JCO.2010.32.7312
Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ et al (2008) A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood 112:3591–3593. https://doi.org/10.1182/blood-2008-02-141598
Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E et al (2011) Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol 12:1195–1203. https://doi.org/10.1016/S1470-2045(11)70243-1
Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B (1996) Graft-versus-myeloma effect: proof of principle. Blood 87:1196–1198
Knop S, Engelhardt M, Liebisch P, Meisner C, Holler E, Metzner B, Deutsche Studiengruppe Multiples Myelom et al (2019) Allogeneic transplantation in multiple myeloma: long-term follow-up and cytogenetic subgroup analysis. Leukemia 33:2710–2719. https://doi.org/10.1038/s41375-019-0537-2
Roos-Weil D, Moreau P, Avet-Loiseau H, Golmard JL, Kuentz M, Vigouroux S, Socie G, Furst S, Soulier J, le Gouill S, Francois S, Thiebaut A, Buzyn A, Maillard N, Yakoub-Agha I, Raus N, Fermand JP, Michallet M, Blaise D, Dhedin N, for the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC), Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) (2011) Impact of genetic abnormalities after allogeneic stem cell transplantation in multiple myeloma: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Haematologica 96:1504–1511. https://doi.org/10.3324/haematol.2011.042713
Rasche L, Röllig C, Stuhler G, Danhof S, Mielke S, Grigoleit GU, Dissen L, Schemmel L, Middeke JM, Rücker V, Schreder M, Schetelig J, Bornhäuser M, Einsele H, Thiede C, Knop S (2016) Allogeneic hematopoietic cell transplantation in multiple myeloma: focus on longitudinal assessment of donor chimerism, extramedullary disease, and high-risk cytogenetic features. Biol Blood Marrow Transplant 22:1988–1996. https://doi.org/10.1016/j.bbmt.2016.08.024
Kröger N, Badbaran A, Zabelina T, Ayuk F, Wolschke C, Alchalby H, Klyuchnikov E, Atanackovic D, Schilling G, Hansen T, Schwarz S, Heinzelmann M, Zeschke S, Bacher U, Stübig T, Fehse B, Zander AR (2013) Impact of high-risk cytogenetics and achievement of molecular remission on long-term freedom from disease after autologous-allogeneic tandem transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant 19:398–404. https://doi.org/10.1016/j.bbmt.2012.10.008
Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA, Chauncey TR, Hari P, Lange T, Pulsipher MA, McSweeney PA, Holmberg L, Becker PS, Green DJ, Mielcarek M, Maloney DG, Storb R (2019) Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica 104:380–391. https://doi.org/10.3324/haematol.2018.200253
Maymani H, Lin P, Saliba RM, Popat U, Bashir Q, Shah N, Patel K, Parmar S, Kebriaei P, Hosing C, Ciurea S, Andersson B, Shpall E, Champlin R, Srour SA, Qazilbash MH (2019) Comparison of outcomes of allogeneic hematopoietic cell transplantation for multiple myeloma using three different conditioning regimens. Biol Blood Marrow Transplant 25:1039–1044. https://doi.org/10.1016/j.bbmt.2019.01.009
Gonsalves WI, Buadi FK, Ailawadhi S, Bergsagel PL, Chanan Khan AA, Dingli D, Dispenzieri A, Fonseca R, Hayman SR, Kapoor P, Kourelis TV, Lacy MQ, Larsen JT, Muchtar E, Reeder CB, Sher T, Stewart AK, Warsame R, Go RS, Kyle RA, Leung N, Lin Y, Lust JA, Russell SJ, Zeldenrust SR, Fonder AL, Hwa YL, Hobbs MA, Mayo AA, Hogan WJ, Rajkumar SV, Kumar SK, Gertz MA, Roy V (2019) Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Bone Marrow Transplant 54:353–367. https://doi.org/10.1038/s41409-018-0264-8
Moreau P, San Miguel JF, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H et al (2017) ESMO Guidelines Committee. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv52–iv61. https://doi.org/10.1093/annonc/mdx096
Gay F, Engelhardt M, Terpos E, Wäsch R, Giaccone L, Auner HW, Caers J, Gramatzki M, van de Donk N, Oliva S, Zamagni E, Garderet L, Straka C, Hajek R, Ludwig H, Einsele H, Dimopoulos M, Boccadoro M, Kröger N, Cavo M, Goldschmidt H, Bruno B, Sonneveld P (2018) From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica 103:197–211. https://doi.org/10.3324/haematol.2017.174573
Sobh M, Michallet M, Gahrton G, Iacobelli S, van Biezen A, Schönland S, Petersen E, Schaap N, Bonifazi F, Volin L, Meijer E, Niederwieser D, el-Cheikh J, Tabrizi R, Fegeux N, Finke J, Bunjes D, Cornelissen J, Einsele H, Bruno B, Potter M, Fanin R, Mohty M, Garderet L, Kröger N (2016) Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia 30:2047–2054. https://doi.org/10.1038/leu.2016.101
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S, Lahuerta JJ, Facon T, Bringhen S, Gay F, Attal M, Passera R, Spencer A, Offidani M, Kumar S, Musto P, Lonial S, Petrucci MT, Orlowski RZ, Zamagni E, Morgan G, Dimopoulos MA, Durie BGM, Anderson KC, Sonneveld P, San Miguel J, Cavo M, Rajkumar SV, Moreau P (2015) Revised International Staging System for Multiple Myeloma: a report from International Myeloma Working Group. J Clin Oncol 33:2863–2869. https://doi.org/10.1200/JCO.2015.61.2267
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, Blaise D, Lowski R, Horowitz M (2009) Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15:1628–1633. https://doi.org/10.1016/j.bbmt.2009.07.004
Shimoni A, Labopin M, Savani B, Hamladji RM, Beelen D, Mufti G, Socié G, Delage J, Blaise D, Chevallier P, Forcade E, Deconinck E, Mohty M, Nagler A (2018) Intravenous busulfan compared with treosulfan-based conditioning for allogeneic stem cell transplantation in acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 24:751–757. https://doi.org/10.1016/j.bbmt.2017.12.776
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus conference on AGvHD grading. Bone Marrow Transplant 15:825–828
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW et al (2015) National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21:389–401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001
Kumar S (2016) International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328–e346. https://doi.org/10.1016/S1470-2045(16)30206-6
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG et al (2015) Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 125:1333–1338. https://doi.org/10.1182/blood-2014-10-609032
Iacobelli S (2013) Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 48:S1–S37. https://doi.org/10.1038/bmt.2012.282
R Foundation for Statistical Computing, Vienna, Austria. R: a language and environment for statistical computing. URL https://www.R-project.org/.
Casper J, Wolff D, Knauf W, Blau IW, Ruutu T, Volin L, Wandt H, Schäfer-Eckart K, Holowiecki J, Giebel S, Aschan J, Zander AR, Kröger N, Hilgendorf I, Baumgart J, Mylius HA, Pichlmeier U, Freund M (2010) Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol 28:3344–3351. https://doi.org/10.1200/JCO.2009.23.3429
Giaccone L, Evangelista A, Patriarca F, Sorasio R, Pini M, Carnevale-Schianca F, Festuccia M, Brunello L, Zallio F, Maffini E, Omedé P, Bringhen S, Mordini N, Fanin R, Ciccone G, Boccadoro M, Bruno B (2018) Impact of new drugs on the long-term follow-up of upfront tandem autograft-allograft in multiple myeloma. Biol Blood Marrow Transplant 24:189–193. https://doi.org/10.1016/j.bbmt.2017.09.017
Passera R, Pollichieni S, Brunello L, Patriarca F, Bonifazi F, Montefusco V, Falda M, Montanari M, Guidi S, Giaccone L, Mordini N, Carella AM, Bavaro P, Milone G, Benedetti F, Ciceri F, Scimè R, Benedetti E, Castagna L, Festuccia M, Rambaldi A, Bacigalupo A, Corradini P, Bosi A, Boccadoro M, Bandini G, Fanin R, Bruno B (2013) Allogeneic hematopoietic cell transplantation from unrelated donors in multiple myeloma: study from the Italian Bone Marrow Donor Registry. Biol Blood Marrow Transplant 19:940–948. https://doi.org/10.1016/j.bbmt.2013.03.012
Franssen LE, Raymakers RA, Buijs A, Schmitz MF, van Dorp S, Mutis T et al (2016) Outcome of allogeneic transplantation in newly diagnosed and relapsed/refractory multiple myeloma: long-term follow-up in a single institution. Eur J Haematol 97:479–488. https://doi.org/10.1111/ejh.12758
Costa LJ, Iacobelli S, Pasquini MC, Modi R, Giaccone L, Blade J, Schonland S, Evangelista A, Perez-Simon JA, Hari P, Brown EE, Giralt SA, Patriarca F, Stadtmauer EA, Rosinol L, Krishnan AY, Gahrton G, Bruno B (2020) Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transplant. 55:1810–1816. https://doi.org/10.1038/s41409-020-0887-4
Chhabra S, Szabo A, Glisch C, George G, Narra RK, Harrington A, Jerkins JH, D'Souza A, Dhakal B, Pasquini MC, Hamadani M, Hari PN (2020) Relapse after allogeneic hematopoietic cell transplantation for multiple myeloma: survival outcomes and factors influencing them. Biol Blood Marrow Transplant. 26:1288–1297. https://doi.org/10.1016/j.bbmt.2020.02.020
López-Corral L, Caballero-Velázquez T, López-Godino O, Rosiñol L, Pérez-Vicente S, Fernandez-Avilés F, Krsnik I, Morillo D, Heras I, Morgades M, Rifon JJ, Sampol A, Iniesta F, Ocio EM, Martin J, Rovira M, Cabero M, Castilla-Llorente C, Ribera JM, Torres-Juan M, Moraleda JM, Martinez C, Vázquez A, Gutierrez G, Caballero D, San Miguel JF, Mateos MV, Pérez-Simón JA (2019) Response to novel drugs before and after allogeneic stem cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transplant 25:1703–1712. https://doi.org/10.1016/j.bbmt.2019.04.026
Pérez-Simón JA, Martino R, Alegre A, Tomás JF, De Leon A, Caballero D et al (2003) Chronic but not acute graft-versus-host disease improves outcome in multiple myeloma patients after non-myeloablative allogeneic transplantation. Br J Haematol 121:104–108. https://doi.org/10.1046/j.1365-2141.2003.04237.x
Gerull S, Goerner M, Benner A, Hegenbart U, Klein U, Schaefer H, Goldschmidt H, Ho AD (2005) Long-term outcome of nonmyeloablative allogeneic transplantation in patients with high-risk multiple myeloma. Bone Marrow Transplant 36:963–969. https://doi.org/10.1038/sj.bmt.1705161
Patriarca F, Einsele H, Spina F, Bruno B, Isola M, Nozzoli C, Nozza A, Sperotto A, Morabito F, Stuhler G, Festuccia M, Bosi A, Fanin R, Corradini P (2012) Allogeneic stem cell transplantation in multiple myeloma relapsed after autograft: a multicenter retrospective study based on donor availability. Biol Blood Marrow Transplant 18:617–626. https://doi.org/10.1016/j.bbmt.2011.07.026
Htut M, D'Souza A, Krishnan A, Bruno B, Zhang MJ, Fei M, Diaz MA, Copelan E, Ganguly S, Hamadani M, Kharfan-Dabaja M, Lazarus H, Lee C, Meehan K, Nishihori T, Saad A, Seo S, Ramanathan M, Usmani SZ, Gasparetto C, Mark TM, Nieto Y, Hari P (2018) Autologous/allogeneic hematopoietic cell transplantation versus tandem autologous transplantation for multiple myeloma: comparison of long-term postrelapse survival. Biol Blood Marrow Transplant 24:478–485. https://doi.org/10.1016/j.bbmt.2017.10.024
Hayden PJ, Iacobelli S, Pérez-Simón JA, van Biezen A, Minnema M, Niittyvuopio R et al (2020) Conditioning-based outcomes after allogeneic transplantation for myeloma following a prior autologous transplant (1991-2012) on behalf of EBMT CMWP. Eur J Haematol 104:181–189. https://doi.org/10.1111/ejh.13352
Schmidt-Hieber M, Blau IW, Trenschel R, Andreesen R, Stuhler G, Einsele H, Kanz L, Keilholz U, Marinets O, Beelen DW, Fauser AA, Volin L, Ruutu T, Uharek L, Fietz T, Knauf W, Hopfenmüller W, Thiel E, Freund M, Casper J (2007) Reduced-toxicity conditioning with fludarabine and treosulfan prior to allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transplant 39:389–396. https://doi.org/10.1038/sj.bmt.1705605
Urbano-Ispizua A, Schmitz N, de Witte T, Frassoni F, Rosti G, Schrezenmeier H et al (2002) Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone Marrow Transplant. 29:639–646. https://doi.org/10.1038/sj.bmt.1703535
Jindal V, Khoury J, Gupta R, Jaiyesimi I (2020) Current status of chimeric antigen receptor T-cell therapy in multiple myeloma. Am J Clin Oncol. 43:371–377. https://doi.org/10.1097/COC.0000000000000669
Shah UA, Mailankody S (2020) CAR T and CAR NK cells in multiple myeloma: expanding the targets. Best Pract Res Clin Haematol. 33:101141. https://doi.org/10.1016/j.beha.2020.101141
Einsele H, Rasche L, Topp MS, Martin Kortüm K, Duell J (2019) The use of bispecific antibodies to optimize the outcome of patients with acute leukemia, lymphoma and multiple myeloma after SCT. Bone Marrow Transplant. 54(Suppl 2):721–726. https://doi.org/10.1038/s41409-019-0596-z
Caballero-Velázquez T, Calderón-Cabrera C, López-Corral L, Puig N, Marquez-Malaver F, Pérez-López E, European Myeloma Network et al (2020) Efficacy of bortezomib to intensify the conditioning regimen and the graft-versus-host disease prophylaxis for high-risk myeloma patients undergoing transplantation. Bone Marrow Transplant. 55:419–430. https://doi.org/10.1038/s41409-019-0670-6
Wolschke C, Stübig T, Hegenbart U, Schönland S, Heinzelmann M, Hildebrandt Y et al (2013) Postallograft lenalidomide induces strong NK cell-mediated antimyeloma activity and risk for T cell-mediated GvHD: results from a phase I/II dose-finding study. Exp Hematol 41:134-142.e3. https://doi.org/10.1016/j.exphem.2012.10.004
Kröger N, Zabelina T, Ayuk F, Atanackovic D, Schieder H, Renges H, Zander A (2006) Bortezomib after dose-reduced allogeneic stem cell transplantation for multiple myeloma to enhance or maintain remission status. Exp Hematol. 34:770–775. https://doi.org/10.1016/j.exphem.2006.02.020
Kröger N, Zabelina T, Klyuchnikov E, Kropff M, Pflüger KH, Burchert A, Stübig T, Wolschke C, Ayuk F, Hildebrandt Y, Bacher U, Badbaran A, Schilling G, Hansen T, Atanackovic D, Zander AR (2013) Toxicity-reduced, myeloablative allograft followed by lenalidomide maintenance as salvage therapy for refractory/relapsed myeloma patients. Bone Marrow Transplant. 48:403–407. https://doi.org/10.1038/bmt.2012.142
Code availability
Not applicable
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. This study was supported with research grants by Blood Disease Research Foundation and Signe and Ane Gyllenberg Foundation.
Author information
Authors and Affiliations
Contributions
Sini Luoma, Raija Silvennoinen, Auvo Rauhala, and Anne Nihtinen designed the study. Sini Luoma and Anne Nihtinen collected the data. Sini Luoma and Auvo Rauhala analyzed the data. Sini Luoma, Raija Silvennoinen, Auvo Rauhala, and Anne Nihtinen wrote the manuscript. Sini Luoma, Raija Silvennoinen, Riitta Niittyvuopio, Tapani Ruutu, Liisa Volin, Eeva Martelin, Vesa Lindström, Jouni Heiskanen, and Anne Nihtinen treated the patients. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval was waived by the local institutional review board of Helsinki University Hospital in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Consent to participate
Informed consent was obtained from all patients for EBMT reporting.
Consent for publication
All the authors approved the manuscript and gave their consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luoma, S., Silvennoinen, R., Rauhala, A. et al. Long-term outcome after allogeneic stem cell transplantation in multiple myeloma. Ann Hematol 100, 1553–1567 (2021). https://doi.org/10.1007/s00277-021-04514-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04514-y