Abstract
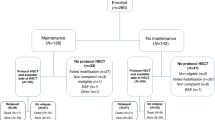
Clinical trials involving various treatment schedules for rituximab maintenance have been conducted for patients with follicular lymphoma (FL) and have not confirmed their impact on serum immunoglobulin (sIg) levels until the completion of maintenance. However, the long-term use of rituximab is a concern because of circulating plasma cell-depletion risk, suggesting that the mechanism of change in sIg levels after RM has not been determined. Additionally, the relationship between host humoral immunity and the prognosis of patients with B cell malignancies has not been determined. We retrospectively investigated data from 213 patients with FL from a single institute who achieved at least a partial response with rituximab, cyclophosphamide, vincristine, and prednisolone with or without doxorubicin. Of these, 166 patients underwent RM with a median period of 1.6 years. A significantly delayed recovery of sIgG levels was observed in the maintenance group until 3 years after RM in comparison to the observation group. A multivariate analysis showed that a sIgG level of < 718 mg/dl 1 year after RM was an independent predictor for poor progression-free survival (PFS) (hazard ratio, 2.3; P = 0.04). Therefore, the sIgG levels scarcely recovered and were significantly delayed after RM, leading to shorter PFS in patients with FL.



Similar content being viewed by others
References
Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR (1994) Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83(2):435–445
Maloney DG, Smith B, Rose A (2002) Rituximab: mechanism of action and resistance. Semin Oncol 2–9(suppl 2):29
Shan D, Ledbetter JA, Press OW (1998) Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood 91(5):1644–1652
Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, Zhang L, Colocci N, Frankel S, Horning SJ (2009) Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 study. J Clin Oncol 27(10):1607–1614. https://doi.org/10.1200/JCO.2008.17.1561
Hainsworth JD, Litchy S, Burris HA et al (2002) Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol 20(20):4261–4267. https://doi.org/10.1200/JCO.2002.08.674
Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, Fey MF, Betticher DC, Martinelli G, Peccatori F, Hess U, Zucca E, Stupp R, Kovacsovics T, Helg C, Lohri A, Bargetzi M, Vorobiof D, Cerny T (2004) Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly 4 schedule. Blood 103(12):4416–4423. https://doi.org/10.1182/blood-2003-10-3411
van Oers MH, Van Glabbeke M, Giurgea L et al (2010) Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 28(17):2853–2858. https://doi.org/10.1200/JCO.2009.26.5827
Taverna CJ, Bassi S, Hitz F et al (2010) Rituximab maintenance treatment for a maximum of 5 years in follicular lymphoma: Safety analysis of the randomized phase III trial SAKK 35/03. Blood 116:1802 1802 (abstract 1802)
Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, Caballero D, Haioun C, Pedersen LM, Delmer A, Simpson D, Leppa S, Soubeyran P, Hagenbeek A, Casasnovas O, Intragumtornchai T, Fermé C, da Silva MG, Sebban C, Lister A, Estell JA, Milone G, Sonet A, Mendila M, Coiffier B, Tilly H (2010) Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 377(9759):42–51. https://doi.org/10.1016/S0140-6736(10)62175-7
van der Kolk LE, Baars JW, Prins MH, van Oers MH (2002) Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood 100(6):2257–2259
Slifka MK, Antia R, Whitmire JK, Ahmed R (1998) Humoral immunity due to long-lived plasma cells. Immunity 8(3):363–372. https://doi.org/10.1016/S1074-7613(00)80541-5
Cooper N, Arnold DM (2010) The effect of rituximab on humoral and cell mediated immunity and infection in the treatment of autoimmune in the treatment of autoimmune disease. Br J Harmatol 149(1):3–13. https://doi.org/10.1111/j.1365-2141.2010.08076.x
Cavanillas F, Liboy I, Pavia O et al (2006) High incidence of non-neutropenic infections induced by rituximab plus fludarabine and associated with hypogammaglobulinemia: a frequently unrecognized and easily treatable complication. Ann Oncol 17(9):1424–1427. https://doi.org/10.1093/annonc/mdl141
Casulo C, Maragulia J, Zelenetz AD (2013) Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk 13(2):106–111. https://doi.org/10.1016/j.clml.2012.11.011
Tsutsumi Y, Kanamori H, Mori A, Tanaka J, Asaka M, Imamura M, Masauzi N (2005) Reactivation of hepatitis B virus with rituximab. Expert Opin Drug Saf 4(3):599–608. https://doi.org/10.1517/14740338.4.3.599
Nishio M, Fujimoto K, Yamamoto S, Endo T, Sakai T, Obara M, Kumano K, Minauchi K, Yamaguchi K, Takeda Y, Sato N, Koizumi K, Mukai M, Koike T (2006) Hypogammaglobulinemia with delayed recovery in memory B cells and an impaired isotype expression after expression after rituximab administration as an adjuvant to autologous stem cell transplantation for non-Hodgkin lymphoma. Eur J Haematol 77(3):226–232. https://doi.org/10.1111/j.1600-0609.2006.00693.x
Irie E, Shirota Y, Suzuki C, Tajima Y, Ishizawa K, Kameoka J, Harigae H, Ishii T (2010) Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int J Hematol 91(3):501–508. https://doi.org/10.1007/s12185-010-0528-6
Cooper N, Davies EG, Thrasher AJ (2009) Repeated courses of rituximab for autoimmune cytopenias may precipitate profound hypogammaglobulinaemia requiring replacement intravenous immunoglobulin. Br J Haematol 146(1):120–122. https://doi.org/10.1111/j.1365-2141.2009.07715.x
Levy R, Mahévas M, Galicier L, Boutboul D, Moroch J, Loustau V, Guillaud C, Languille L, Fain O, Bierling P, Khellaf M, Michel M, Oksenhendler E, Godeau B (2014) Profound symptomatic hypogammaglobulinemia: a rare late complication after rituximab treatment for immune thrombocytopenia. Report of 3 cases and systematic review of the literature. Autoimmun Rev 13(10):1055–1063. https://doi.org/10.1016/j.autrev.2014.08.036
Finn OJ (2008) Cancer immunology. N Engl J Med 358(25):2704–2715. https://doi.org/10.1056/NEJMra072739
Rescigno M, Avogadri F, Curigliano G (2007) Challenges and prospects of immunotherapy as cancer treatment. Biochim Biophys Acta 1776(1):108–123. https://doi.org/10.1016/j.bbcan.2007.07.003
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Reuschenbach M, von Knebel Doeberitz M, Wentzensen N (2009) A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother 58(10):1535–1544. https://doi.org/10.1007/s00262-009-0733-4
Houot R, Levy R (2009) Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev 23(3):137–142. https://doi.org/10.1016/j.blre.2008.09.001
Pillai S, Mattoo H, Cariappa A (2011) B cells and autoimmunity. Curr Opin Immunol 23(6):721–731. https://doi.org/10.1016/j.coi.2011.10.007
Swerdlow SH, Campo E, Harris NL et al (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon. IARC Press, Lyon
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586. https://doi.org/10.1200/JCO.2006.09.2403
Coiffier B, Lepage E, Briere J et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large- B-cell lymphoma. N Engl J Med 346(4):235–242. https://doi.org/10.1056/NEJMoa011795
Hochster HS, Weller E, Gascoyne R et al (2007) Cyclophosphamide/fludarabine (CF) in advanced indolent lymphoma: results from the E1496 Trial. J Clin Oncol 25(18):8004 8004 (abstract 8004)
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Lu H, Pundole X (2014) Factors predicting long-term hypogammaglobulinemia in lymphoma survivors. J Allergy Clin Imunol 44(2):AB10
Ng PP, Jia M, Patel KG, Brody JD, Swartz JR, Levy S, Levy R (2012) A vaccine directed to B cells and produced by cell-free protein synthesis generates potent antilymphoma immunity. Proc Natl Acad Sci U S A 109(36):14526–14531. https://doi.org/10.1073/pnas.1211018109
Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R (2004) Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol 22(23):4717–4724. https://doi.org/10.1200/JCO.2004.06.003
Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R (1992) Induction of immune responses in patients with B-cell lymphoma against the surface immunoglobulin idiotype expressed by their tumors. New Engl J Med 327(17):1209–1215. https://doi.org/10.1056/NEJM199210223271705
Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, Watson TM, Reynolds CW, Gause BL, Duffey PL, Jaffe ES, Creekmore SP, Longo DL, Kwak LW (1999) Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony stimulating factor against lymphoma. Nat Med 5(10):1171–1177. https://doi.org/10.1038/13928
Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R (1996) Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 2(1):52–58. https://doi.org/10.1038/nm0196-52
Hsu FJ, Caspar CB, Czerwinski D, Kwak LW, Liles TM, Syrengelas A, Taidi-Laskowski B, Levy R (1997) Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma—long-term results of a clinical trial. Blood 89(9):3129–3135
Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, van Beckhoven A, Liles TM, Engleman EG, Levy R (2002) Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood 99(5):1517–1526. https://doi.org/10.1182/blood.V99.5.1517
Levy R, Robertson MJ, Ganjoo K, et al. Results of a phase 3 trial evaluating safety and efficacy of specific immunotherapy, recombinant idiotype (Id) conjugated to KLH (Id-KLH) with GM-CSF, compared to nonspecific immunotherapy, KLH with GM-CSF, in patients with follicular non-Hodgkin’s lymphoma (fNHL). AACR meeting; 2008 Abstract LB-204
Ai WZ, Tibshirani R, Taidi B et al (2009) Anti-idiotype antibody response after vaccination correlates with better overall survival in follicular lymphoma. Blood 113:5743–5746
Bertinetti C, Zirlik K, Heining-Mikesch K, Ihorst G, Dierbach H, Waller CF, Veelken H (2006) Phase I trial of a novel intradermal idiotype vaccine in patients with advanced B-cell lymphoma: specific immune responses despite profound immunosuppression. Cancer Res 66(8):4496–4502. https://doi.org/10.1158/0008-5472.CAN-05-4233
Redfern CH, Guthrie TH, Bessudo A, Densmore JJ, Holman PR, Janakiraman N, Leonard JP, Levy RL, Just RG, Smith MR, Rosenfelt FP, Wiernik PH, Carter WD, Gold DP, Melink TJ, Gutheil JC, Bender JF (2006) Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin’s lymphoma resulting in durable clinical responses. J Clin Oncol 24(19):3107–3112. https://doi.org/10.1200/JCO.2005.04.4289
Flinn I, Van der Jagt R, Chang JE et al (2017) First-line treatment of iNHL or MCL patients with BR or R-CHOP/R-CVP: Results of the BRIGHT 5-year follow-up study. J Clin Oncol 35(15 suppl):7500–7500
Rummel MJ, Maschmeyer G, Ganser A et al (2017) Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: Nine-year updated results from the StiL NHL1 study. J Clin Oncol 35(15 suppl):7501–7501
Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haïoun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL, Zucca E, Montserrat E (2004) Follicular lymphoma international prognostic index. Blood 104(5):1258–1265. https://doi.org/10.1182/blood-2003-12-4434
Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran P, Cortelazzo S, Martinelli G, Martelli M, Rigacci L, Arcaini L, di Raimondo F, Merli F, Sabattini E, McLaughlin P, Solal-Céligny P (2009) Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 27(27):4555–4562. https://doi.org/10.1200/JCO.2008.21.3991
Acknowledgements
The authors thank Chizuru Suitzu for data management and Sayuri Minowa for tissue management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The institutional review board of the Cancer Institute Hospital in Tokyo, Japan approved the study, which was conducted in accordance with the Declaration of Helsinki.
Conflict of interest
All authors except the following four have no conflict of interest to declare:
Yasuhito Terui has received honoraria from Janssen. Masahiro Yokoyama, Yuko Mishima, and Noriko Nishimura have received consultancy from Chugai. Kiyohiko Hatake has received consultancy from Meiji-Seika and Otsuka, honoraria from Kyowa Kirin, and research funding from Chugai and Kyowa Kirin.
Electronic supplementary material
Supplemental Figure 1
Kaplan–Meier curves showing the survival of patients with follicular lymphoma from the maintenance group and observation group. (A) progression-free survival, (B) time to next treatment, and (C) overall survival (GIF 15 kb)
High resolution image
(EPS 464 kb)
Supplemental Figure 2
Variation of sIgG levels from the last therapy to 1 and 2 years later in the observation group and before and after the maintenance phase in the maintenance group. (GIF 13 kb)
High resolution image
(EPS 153 kb)
Rights and permissions
About this article
Cite this article
Kusano, Y., Yokoyama, M., Inoue, N. et al. Delayed recovery of serum immunoglobulin G is a poor prognostic marker in patients with follicular lymphoma treated with rituximab maintenance. Ann Hematol 97, 289–297 (2018). https://doi.org/10.1007/s00277-017-3175-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3175-7