Abstract
Introduction
Allogeneic hematopoietic cell transplantation (HCT) was a standard therapy in chronic phase (CP) chronic myeloid leukemia (CML). As a result of the effective therapy with tyrosine kinase inhibitors (TKI), HCT was shifted to defined clinical situations. We present the results of observational prospective analysis of 28 CML patients undergoing HCT after exposure to, at least, two lines of TKI (including dasatinib and/or nilotinib), with respect to response, overall survival (OS), treatment toxicity, graft versus host disease (GVHD), and progression/relapse incidence.
Results
All the patients but one engrafted with median time 19 days. OS for patients in CP1 and CP2/accelerated phase (AcP) were 92.9 and 85.7 %, respectively. Six patients allotransplanted in blast crisis (BC) CML died early after HCT.
Eighteen patients achieved deep molecular remission (MR4.5 or MR4.0). Relapse incidence was 29.6 %. Median time to progression (TTP) differs significantly depending on the CML phase prior to HCT, the best response achieved after HCT and development of chronic GvHD.
NRM yielded the values 7.1, 12.5, and 50 % in CP1, CP2/AcP, and BC, respectively. Fatal outcome, due to veno-occlusive disease (VOD), was observed in two (7 %) patients. In five (17.9 %) patients, mild or moderate VOD was observed with no negative impact of preceding therapy with TKI2. Acute GvHD was diagnosed in 25.9 % of patients, while chronic GvHD developed in 42.9 % of individuals.
Conclusion
Pretransplantation therapy with TKI2 in CP CML is safe and reasonable. In BC, the optimal approach before HCT is to reduce the leukemic burden and achieve subsequent CP.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic cell transplantation (HCT) was historically a standard curative therapy for patients with chronic phase (CP) chronic myeloid leukemia (CML). As a result of the novel highly effective therapy with tyrosine kinase inhibitors (TKI), the use of HCT was shifted to defined clinical situations. Following the European LeukemiaNet (ELN) guidelines, patients with Philadelphia positive (Ph+) CML diagnosed in the advanced phase (accelerated phase (AcP); blast phase/crisis (BC)) or resistant to imatinib or second-generation TKI (TKI2), should be qualified to HCT [1]. A number of published data shows no serious negative impact of imatinib mesylate therapy prior to HCT [2–5], but only few studies concerning the outcome of HCT in TKI2-resistant patients are currently available [6].
Non-relapse mortality (NRM) and organ toxicity in patients receiving TKI2 before transplantation are of interest. Based on the toxicity profile of both dasatinib and nilotinib, development of hepatic veno-occlusive disease (VOD), cardiac toxicity, as well as immune dysfunctions or delayed engraftment can be expected [7, 8]. The long term outcome of TKI2-resistant patients remains another important issue. Potentially poorer outcome of patients pre-treated with TKI2 may result from the selection of more aggressive clones of malignant cells with additional molecular aberrations, promoting their proliferation and survival abilities. Moreover, TKI2-resistant patients are usually transplanted in more advanced phases of the disease or are simply older, when they finally enter the procedure of HCT. Additional problems are related to the late toxicity of HCT and chronic GvHD, significantly affecting the quality of life.
In this study, we present the results of observational prospective analysis of CML patients undergoing allogeneic hematopoietic cell transplantation after exposure to TKI2, as the second-line therapy, with respect to response, overall survival, treatment toxicity, GVHD and progression/relapse incidence.
Patients and methods
Patients
The eligible subjects were patients undergoing allogeneic hematopoietic cell transplantation with CML diagnosis, after pretreatment with imatinib mesylate and one or more TKI2. The therapy with TKI2 was discontinued at least 2 days prior to the conditioning regimen. Treatment was provided in two transplant centers, between February 2008 and November 2013.
The study was approved by the Bioethical Committee of Medical University of Gdansk and was conducted in accordance with the Declaration of Helsinki.
Definitions
Chronic phase CML was diagnosed according to ELN criteria when blasts in peripheral blood (PB) and bone marrow (BM) were <15 %, basophilia did not exceed 20 %, and there was no evidence of extramedullary involvement. Accelerated phase was defined by blasts between 15 and 29 %, basophilia exceeding 20 %, persistent thrombocytopenia (<100 G/l, not related to the treatment) or thrombocytosis unresponsive to the therapy or any cytogenetic evidence of clonal evolution. Blast phase/crisis was diagnosed when there were 30 % or more PB or BM blasts, or the extramedullary blast proliferation occurred.
Molecular monitoring was performed according to the ELN guidelines with the RQ-PCR method [9–11]. Deep molecular response MR4.5 was defined as a 4.5-log reduction from the baseline (≤0.0032 % BCR-ABL [IS]) or a disease undetectable in complementary DNA (cDNA) with the amount of the transcript ABL ≥32.000. Deep MR4.0 was defined as a 4-log reduction from the baseline (≤0.01 % BCR-ABL [IS]) or a disease, which was undetectable in cDNA with the amount of the transcript ABL ≥10.000. Major molecular response (MMR) was determined when there was a 3-log reduction from the baseline (≤0.1 % BCR-ABL [IS]). Minor molecular response (MiMR) was the response below MMR.
Progression/relapse of CML after HCT was defined by any hematologic, cytogenetic, or molecular evidence of the disease, requiring the additional treatment after HCT (e.g., TKI re-administration and/or donor lymphocyte infusion (DLI)).
Engraftment was achieved when an absolute neutrophil count was greater or equal to 0.5 G/l, and it was the first of three consecutive days with this value.
Acute graft versus host disease (GvHD) was diagnosed according to IBMTR criteria [12, 13]. The diagnosis of chronic GvHD was based on NIH consensus criteria [14].
Hepatic veno-occlusive disease (VOD) was diagnosed with occurrence of at least two modified Seattle criteria: hyperbilirubinemia (>2 mg/dl), hepatomegaly with right upper quadrant abdominal pain, and >2 % weight gain due to fluid retention [15].
NRM was defined as any case of death without symptoms of relapse.
Statistical analysis
Differences in median values between groups were analyzed with the Mann-Whitney U test. Correlations were analyzed using the Spearman’s rank test. Stratified Kaplan-Meier curves were applied to evaluate association between patient characteristics and the outcomes. Log-rank test was used to compare the curves. Since for most of the variables the median survival was not achieved, we reported the mean survival time restricted to the longest follow-up time. For most of the variables, the largest observed analysis time was censored and underestimation of the restricted mean survival time was expected. Multivariate analysis was performed using the Cox regression. P values <0.05 were considered statistically significant. Calculations were performed using the Stata 13.1 statistical software (StataCorp, TX, USA).
Results
We prospectively analyzed the data of 28 patients treated with the second generation TKI (dasatinib, nilotinib, or both in sequence) before HCT. None of the studied patients, including those with BC, received conventional chemotherapy prior to HCT. Their characteristics are presented in Table 1. Patients were followed-up up to February 2014.
Engraftment
All the patients, except for one in BC, engrafted with median time 19 days (13–43 days). The duration of TKI2 therapy prior to HCT had no impact on the time of engraftment (p = 0.41).
Response
Eighteen patients achieved MR4.5 or MR4.0 with median time 1.8 months after HCT, and this response was maintained in 14 alive patients. Five patients achieved MMR, as the best molecular response, with median time 1.1 months after HCT, and this group included one patient in early post-transplant period, one who died due to aGvHD and three patients who lost this response. Four patients achieved responses below MMR (MiMR) and all of them died, due to relapse or aGvHD.
The data concerning the time to achieve the best molecular response were available for 27 patients. The median value was 1.8 months after HCT and did not depend on patients’ gender (p = 0.4), age (p = 0.98), type of a donor (p = 0.23), and the source of hematopoietic cells (p = 0.25). Median time to achieve the best molecular response was the shortest for patients in CP1 (1.1 months) and the longest in BC CML at HCT (2.4 months) but the trend was not statistically significant (p = 0.35). The best molecular response was not influenced by aGvHD occurrence (p = 0.52) or cGvHD development (p = 0.96).
Survival
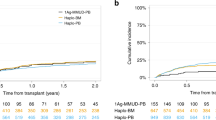
During median 19 months (3–66) of follow-up, 19 patients survived. Deaths were related to VOD, aGvHD and relapse. Since all the deaths occurred within the first year after HCT, the 1- and 3-year observed survival rates were identical and yielded a value of 71.4 %. OS did not depend on patients’ gender (p = 0.97), age (p = 0.11), type of a donor (p = 0.97), and the source of hematopoietic cells (p = 0.83) but was strongly influenced by the CML phase prior to HCT (p = 0.0001), Fig. 1.
Crude OS estimates significantly depended on the best molecular response achieved after HCT; however, the result was confounded by the CML phase at HCT. After adjustment for the confounder, patients with deep MR had better prognosis, but the effect was not statistically significant (HR = 0.1; p = 0.08).
Kaplan-Meier estimates of OS for patients in CP1 and CP2/AcP were 92.9 and 85.7 %, respectively. In the group of 14 patients with CP1 one patient died due to acute GvHD (grade IV). Among eight patients with more advanced phase (CP2 or Ac): one died because of aGvHD (grade IV) and one allotransplanted from the family donor relapsed early in BC CML.
Six patients were allotransplanted in BC. All of them died in the early post-transplant period: three due to relapse, one as a result of fatal acute GvHD, one because of VOD and one due to multiorgan failure (MOF)/VOD.
Time to progression
Median time to progression (TTP) in the whole study group has not been achieved yet, while the mean time has reached 45 months. Median TTP in the group of patients, who lost their molecular response was 5 months.
TTP did not depend on patients’ gender (p = 0.63), age (p = 0.98), type of a donor (p = 0.22), the source of hematopoietic cells (p = 0.55), and occurrence of aGvHD (p = 0.55) but differs significantly depending on CML phase prior to HCT (p = 0.046), Fig. 2a. TTP also differs depending on the level of response achieved after HCT (p = 0.009), Fig. 2b. Moreover, TTP was significantly influenced by the development of cGvHD (p = 0.038), Fig. 2c.
a–c Impact of clinical factors on the incidence of relapse. Cumulative rates of relapses were estimated according to the competing risk method. Non-relapse mortality was evaluated as a competing risk. CP chronic phase, Ac accelerated phase, BC blast crisis/phase, CMR complete molecular response, MMR major molecular response, MiMR minor molecular response
Relapse incidence
Relapse after HCT was seen in eight patients (29.6 %). The incidence of relapse in CP1, CP2/Ac, and BC was 21.4, 12.5, and 50 %, respectively. Altogether, three molecular relapses and five hematological relapses were diagnosed. Hematological relapse was a direct cause of death in three cases. Those patients were in BC prior to transplantation and had achieved at most MMR after HCT. Among the remaining four relapsed patients, three were transplanted in CP1 and one in CP2. All four patients received RIC prior to HCT. They were qualified to DLI-based therapy: three due to molecular relapses and one due to hematological relapse (combined treatment with DLI and dasatinib). In all of them deep MR is maintained until the end of follow-up.
Treatment toxicity and GVHD
Total incidence of VOD was 25 % (seven patients) in the study group. Fatal outcome due to severe transplant-related toxicity was seen in two (7.1 %) patients, both in BC: hepatic VOD and MOF/VOD. In five (17.9 %) patients, mild or moderate VOD was observed with complete recovery after conventional treatment, including two patients in BC and three patients in CP1/CP2/Ac phase CML. The incidence of VOD in BC and CP1/CP2/Ac phase was 66.7 and 13.6 %, respectively. A prolonged therapy with TKI2 had no influence on VOD occurrence. Incidence of VOD development decreased along with the duration of TKI2 treatment (p = 0.019).
Acute graft versus host disease (aGvHD) was diagnosed in 7 out of 27 patients (25.9 %) including grades I–II in four patients (11 %). It was established in one (3.7 %) patient in CP1, three (11.1 %) patients in CP2/Ac, and three (11.1 %) patients in BC CML. Median survival of patients with aGvHD grade IV was 3 months.
Among 21 patients with the follow-up period exceeding 100 days after HCT, chronic GvHD (cGvHD) developed in nine (42.9 %) patients, including one person with cGvHD induced by DLI. In five (21 %) mild and in four cases, moderate cGvHD was observed. Chronic GvHD had no impact on OS (p = 0.18).
NRM reached 7.1 % for CP1, 12.5 % for CP2/AcP, and 50 % for BC and included deaths caused by VOD/MOF and GvHD-related complications.
Discussion
The therapy with tyrosine kinase inhibitors has been a standard care for patients with CML for more than one decade; that is why data concerning the results of HCT performed nowadays in TKI-naive individuals are no longer available. Due to the advance in post-transplant care, our results cannot be compared reliably to a historical group, prior to the era of TKI, but might be compared to the published data concerning the imatinib-pretreated group of patients. The concept to compare data comes from the similarities in the toxicity profile of imatinib and TKI2.
The clinically important finding of our research is a lack of severe hepatic VOD and engraftment failure that could potentially complicate the use of second-generation TKI prior to HCT in a group of patients in early (CP1) or advanced (CP2 and AcP) phase of CML. The therapy with dasatinib or nilotinib in our study has been discontinued before the conditioning regimen started, the same as imatinib in published studies. Zaucha et al. [5] presented the results of transplantation in the group of 30 patients receiving imatinib prior to HCT, including 26 patients with CML. The group characteristics differ with the older median age (48 vs 32 years), a smaller number of patients transplanted from BM and a higher proportion of patients in BC CML in our study group. Graft failure was reported in two patients in the imatinib group and in one patient in the TKI2 group, while median time to engraftment after PBSCT was comparable in both groups. To confront hepatotoxicity, 23 % of patients in the imatinib group experienced severe hyperbilirubinemia including 10 % who met VOD criteria, while in our TKI2 group, 25 % developed VOD, including 7.1 % with fatal outcome, however observed in patients with BC CML. Patients in CP1/CP2 in our study had VOD incidence about 14 %, that is comparable to cumulative incidence of VOD recently reported as about 13.8 % using Seattle criteria [16], and there was no VOD/MOF-related death in this group. Moreover, a prolonged TKI2 therapy, reducing the leukemia burden, was associated with lower incidence of hepatic toxicity. Therefore, the strategy to continue TKI2 treatment as long as it is necessary to find a donor is a reasonable tactic and TKI2 tapering few days prior to conditioning appears to be the safe approach.
In contrast to transplantation performed in CP1, CP2, or AcP phase, BC CML increases significantly the risk of severe complications and relapse after HCT, which is in line with data reported by other authors [17, 18]. It is essential to reduce the load of leukemic cells by TKI and/or chemotherapy to the level of the second or subsequent CP to achieve the long-lasting remission. In contrast to high relapse rate in patients in BC, among seven patients transplanted in CP2 only one patient died due to relapse, one had molecular relapse successfully treated with DLI, and one died due to aGvHD grade IV in deep MR. With this thesis, we suggest not to postpone HCT in case of hematological response to TKI2 while waiting for the deeper molecular response and risking the clonal progression. Moreover, patients with BC at HCT should be attentively and frequently monitored after transplantation with the use of the most sensitive PCR-based tests to detect molecular progression or relapse. In this high-risk group of patients, the use of DLI with re-introduction of TKI, may prevent the disease progression and improve the outcome. It is well known that patients with relapse detected on the molecular level respond to DLI with higher remission rate compared to any more advanced disease [19, 20].
We also analyzed the incidence of acute and chronic GvHD since the immunomodulatory potency of TKI may determine the incidence and severity of GvHD. In case of imatinib, the suggested mechanism of immunosuppression is probably related to inhibition of T cell activation and dendritic cell development [21]. Similar data concerning nilotinib, but mainly based on in vitro studies, have been reported [22]. The efficacy but also toxicity profile of dasatinib is correlated with immunomodulatory activity related to the induction of monoclonal T/NK cell proliferation [23]. It is explained by a broad spectrum of inhibited kinases, including SCR-kinase. Dasatinib has also an ability to block T cell activity, proliferation, secretion, and TCR-signaling pathway [24–26]. The significance of these clonal lymphocytes in anti-leukemic surveillance and their ability to influence graft versus leukemia and GvHD have not been determined. In our TKI2 study group, the incidence of aGvHD was 25.9 % including 14.9 % in grade III-IV and was lower than that reported by Zaucha et al. [5].
Clinically important are encouraging results of survival and response after HCT achieved in patients with failure of TKI2 therapy. OS for 22 patients in CP1 and CP2/AcP were 92.9 and 85.7 %, respectively. Among them 18 patients achieved MR4.5 or MR4.0 with median time 1.8 months after HCT, and this response has been maintained in 14 alive patients. In four patients with relapse, deep MR was restored by DLI with/without TKI support. This confirms the important role of HCT in CML management.
We conclude that in the view of the curative potential of HCT and non-inferior survival of patients in CP CML with the pretransplantation TKI2 therapy, compared to the imatinib therapy, it is a safe and reasonable approach. Nonetheless, further studies are warranted to assess the individual influence of nilotinib and dasatinib on the transplant-related toxicity and the outcome of HCT. In blast crisis, the optimal approach before HCT is to reduce the leukemic burden. Achievement of subsequent CP seems to be sufficient to improve the outcome in this group of patients.
References
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF et al (2013) European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 122(6):872–84
Lee SJ, Kukreja M, Wang T, Giralt SA, Szer J, Arora M et al (2008) Impact of prior imatinib mesylate on the outcome of hematopoietic cell transplantation for chronic myeloid leukemia. Blood 112(8):3500–7
Oehler VG, Gooley T, Snyder DS, Johnston L, Lin A, Cummings CC et al (2007) The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood 109(4):1782–9
Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R et al (2010) Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood 115(10):1880–5
Zaucha JM, Prejzner W, Giebel S, Gooley TA, Szatkowski D, Kalwak K et al (2005) Imatinib therapy prior to myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant 36(5):417–24
Breccia M, Palandri F, Iori AP, Colaci E, Latagliata R, Castagnetti F et al (2010) Second-generation tyrosine kinase inhibitors before allogeneic stem cell transplantation in patients with chronic myeloid leukemia resistant to imatinib. Leuk Res 34(2):143–7
Kim TD, Lle CP, Schwarz M, Grille P, Levitin M, Fateh-Moghadam S et al (2012) Clinical cardiac safety profile of nilotinib. Haematologica 97(6):883–9
Valent P (2011) Severe adverse events associated with the use of second-line BCR/ABL tyrosine kinase inhibitors: preferential occurrence in patients with comorbidities. Haematologica 96(10):1395–7
Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E et al (2003) Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using 'real-time' quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia 17(12):2474–86
Cross NC, White HE, Muller MC, Saglio G, Hochhaus A (2012) Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 26(10):2172–5
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N et al (2003) Standardization and quality control studies of 'real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia 17(12):2318–57
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15(6):825–8
Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ et al (1997) IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol 97(4):855–64
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11(12):945–56
Carreras E (2000) Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol 64(5):281–91
Carreras E, Diaz-Beya M, Rosinol L, Martinez C, Fernandez-Aviles F, Rovira M (2011) The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant 17(11):1713–20
Naik S, Wong R, Arai S, Brown J, Laport G, Lowsky R et al (2011) Long-term outcomes in patients with high-risk myeloid malignancies following matched related donor hematopoietic cell transplantation with myeloablative conditioning of BU, etoposide and CY. Bone Marrow Transplant 46(2):192–9
Wadhwa J, Szydlo RM, Apperley JF, Chase A, Bua M, Marin D et al (2002) Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood 99(7):2304–9
Deol A, Lum LG (2010) Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev 36(7):528–38
Shanavas M, Messner HA, Kamel-Reid S, Atenafu EG, Gupta V, Kuruvilla J et al (2014) A comparison of long-term outcomes of donor lymphocyte infusions and tyrosine kinase inhibitors in patients with relapsed CML after allogeneic hematopoietic cell transplantation. Clin Lymphoma Myeloma Leuk 14(1):87–92
Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC et al (2005) Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 105(6):2473–9
Chen J, Schmitt A, Chen B, Rojewski M, Rubeler V, Fei F et al (2008) Nilotinib hampers the proliferation and function of CD8+ T lymphocytes through inhibition of T cell receptor signalling. J Cell Mol Med 12(5B):2107–18
Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R et al (2010) Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 116(5):772–82
Blake S, Hughes TP, Mayrhofer G, Lyons AB (2008) The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin Immunol 127(3):330–9
Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R et al (2008) Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 111(3):1366–77
Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK et al (2008) Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res 14(8):2484–91
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Piekarska, A., Gil, L., Prejzner, W. et al. Pretransplantation use of the second-generation tyrosine kinase inhibitors has no negative impact on the HCT outcome. Ann Hematol 94, 1891–1897 (2015). https://doi.org/10.1007/s00277-015-2457-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2457-1