Abstract
Purpose
To compare fetal and adult morphologies of the orbital muscle (OM) and to describe the detailed topographical anatomy in adults.
Methods
Using unilateral orbits from 15 near-term fetuses and 21 elderly cadavers, semiserial horizontal or sagittal paraffin sections were prepared at intervals of 20–100 µm. In addition to routine histology, we performed immunohistochemistry for smooth muscle actin.
Results
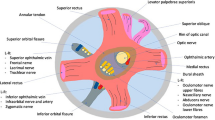
At near term, the OM consistently extended widely from the zygomatic bone or the greater wing of the sphenoid to the maxilla or ethmoid. Thus, it was a large sheet covering the future inferior orbital fissure. In contrast, the adult OM was a thin and small muscle bundle connecting (1) the greater wing of the sphenoid to the maxilla (11/19 cadavers), (2) the lesser wing of the sphenoid to the maxilla (5/19) or the greater wing (3/19). The small OM was likely to be restricted within the greater wing (5/19 cadavers) or the maxilla (3/19). Two of these five types of OM coexisted in eight orbits. OM attachment to the lesser wing was not seen in fetuses, whereas ethmoid attachment was absent in adults.
Conclusions
The lesser wing attachment of the OM seemed to establish after birth. A growing common origin of the three recti was likely involved in “stealing” the near-term OM attachment from the ethmoid. The strong immunoreactivity of remnant-like OM in the elderly suggests that OM contraction is still likely to occur against the increased flow through a thin vein. However, the contraction might have no clinical significance.




Similar content being viewed by others
Abbreviations
- Ala maj:
-
Greater wing of the sphenoid bone
- Ala min:
-
Lesser wing of the sphenoid bone
- ETH :
-
Ethmoid bone
- FB :
-
Frontal bone
- IO :
-
Inferior obliquus muscle
- ION :
-
Infraorbital nerve
- IR :
-
Inferior rectus muscle
- LR :
-
Lateral rectus muscle
- MCF :
-
Middle cranial fossa
- MX :
-
Maxilla
- OM :
-
Orbital muscle
- PPG:
-
Pterygopalatine ganglion
- PT :
-
Pterygoid process
- SP :
-
Sphenoid bone
- TM :
-
Temporalis muscle
- V1 :
-
Ophthalmic nerve
- V2 :
-
Maxillary nerve
- V3 :
-
Mandibular nerve
- ZY :
-
Zygomatic bone
References
Bergen MP (1982) Some histological aspects of the structure of the connective tissue system and its relationships with the blood vessels in the human orbit. Acta Morphol Neerl Scand 20:293–308
Clark RA, Demer JL (2018) Magnetic resonance imaging of the globe-tendon interface for extraocular muscle: is there an Arc of contact? Am J Ophthalmol 194:171–181
De Battista JC, Zimmer LA, Rodríguez-Vázquez JF et al (2011) Muller’s muscle, no longer vestigial in endoscopic surgery. World Neurosurg 76:342–346
de Haan AB, Willekens B, Klooster J et al (2006) The prenatal development of the human orbit. Strabismus 14:51–56
Demer JL, Miller JM, Poukens V et al (1995) Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci 36:1125–1136
Dutton JJ (2011) Atlas of clinical and surgical orbital anatomy. PA, WB Saunders
Hinata N, Murakami G (2014) The urethral rhabdosphincter, levator ani muscle and perineal membrane: a review. BioMedical Res Int. https://doi.org/10.1155/2014/906921
Honkura Y, Yamamoto M, Rodríguez-Vázquez JF et al (2021) Fetal development of the carotid canal with special reference to a contribution of the sphenoid bone and pharyngotympanic tube. Anat Cell Biol54(2):259-269
Jin ZW, Abe H, Jin Y, Shibata S et al (2016) Positional changes in tendon insertions from a bone to fascia: human fetal development of the pes anserinus and the semimembranosus muscle insertion. Folia Morphol 75:503–511
Jordan DR (1992) The orbital muscle of Muller. Arch Ophthalmol 110:1798–1799
Kim JH, Hayashi S, Yamamoto M et al (2020) Examination of the tendinous annulus of Zinn for a common origin of the extraocular recti 2. An embryological basis of extraocular muscle anomalies. Investig Ophthal Vis Sci 61(12):5
Kono R, Poukens V, Demer JL (2002) Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci 43:2923–2932
Koornneef L (1988) Eyelid and orbital fascial attachments and their clinical significance. Eye 2:130–134
Last RJ (1970) Eugene Wolff’s anatomy of the eye and orbit. PA, WB Saunders
Meshida K, Lin S, Domning DP et al (2020) Cetacean orbital muscles: anatomy and function of the circular layers. Anat Rec 303:1792–1811
Miyake N, Hayashi S, Kawase T et al (2010) Fetal anatomy of the human carotid sheath and structures in and around it. Anat Rec 293:438–445
Naito T, Cho KH, Yamamoto M et al (2019) Examination of the topographical anatomy and fetal development of the tendinous annulus of Zinn for a common origin of the extraocular recti. Invest Ophthal Vis Sci 60:4564–4573
Naito M, Suzuki R, Abe H et al (2015) Fetal development of the human obturator internus muscle with special reference to the tendon and pulley. Anat Rec 298(7):1282–1293
Ngnitewe Massa R, Minutello K, Mesfin FB (2020) StatPearls [Internet] neuroanatomy. StatPearls Publishing Cavernous Sinus
Osanai H, Abe S, Rodríguez-Vázquez JF et al (2011) Human orbital muscle: a new point of view from the fetal development of extraocular connective tissue. Investig Ophthalmol Vis Sci 52:1501–1506
Rodríguez-Vázquez JF, Mérida-Velasco JR, Arráez-Aybar LA et al (1998) Anatomic relationships of the orbital muscle of Müller in human fetuses. Surg Radiol Anat 20:341–344
Rodríguez-Vázquez JF, Mérida-Velasco JR, Jiménez-Collado J (1990) Orbital muscle of Müller: observations on human fetuses measuring 35–150 mm. Acta Anat 139:300–303
Sasaki H, Hinata N, Kurokawa K et al (2014) Supportive tissues of the vagina with special reference to a fibrous skeleton in the perineum: a review. Open J Obstet Gynecol 4:144–157
Smith TJ, Hegedus L (2016) Grave’s disease. N Engl J Med 375:1552
Tawfik HA, Dutton JJ (2018) Embryologic and fetal development of the human orbit. Ophthalmic Plast Reconstr Surg 34:405–421
Toerien MJ, Gous AEF (1978) The orbital muscle of Müller. S Afr Med J 53:139–141
Wang Y, Smith TJ (2014) Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Investig Ophthalmol Vis Sci 55:1735–1748
Warwick R (1976) Eugene Wolff’s anatomy of the eye and orbit, 7th edn. Saunders
Williams PL (1995) Gray’s Anatomy, 38th ed. Churchill Livingstone, p 1353
Xu L, Li L, Xie C et al (2017) Thickness of extraocular muscle and orbital fat in MRI predicts response to glucocorticoid therapy in Graves’ ophthalmopathy. Int J Endocrinol. https://doi.org/10.1155/2017/3196059
Yamamoto M, Takada H, Ishizuka S et al (2020) Morphological association between the muscles and bones in the craniofacial region. PLoS ONE 15(1):e0227301
Yang JD, Ishikawa K, Hwang HP et al (2013) Morphology of the ligament of Treitz likely depends on its fetal topographical relationship with the left adrenal and liver caudate lobe as well as developing lymphatic tissues: a histological study using human fetuses. Surg Radiol Anat 35:25–38
Acknowledgements
This study was supported by Wonkwang University in 2020.
Author information
Authors and Affiliations
Contributions
KC: conceptualization and manuscript writing, ZJ: formal analysis and investigation, SU: methodology and literature research, MY: data collection and conceptualization. GM: conceptualization and manuscript writing. SA: management and conceptualization. JV: conceptualization and data collection.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to be declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, K.H., Jin, Z.W., Umeki, S. et al. Human orbital muscle in adult cadavers and near-term fetuses: its bony attachments and individual variation identified by immunohistochemistry. Surg Radiol Anat 43, 1813–1821 (2021). https://doi.org/10.1007/s00276-021-02819-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-021-02819-1