Abstract
Purpose
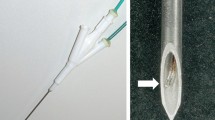
To investigate the role of a novel curved radiofrequency ablation (RFA) electrode with controllable direction in the ablation of tumors behind large hepatic vessels in ex vivo bovine and in vivo canine liver experiments.
Materials and Methods
Approval from the institutional animal care and use committee was obtained. In ex vivo experiments, conventional multi-tines expandable electrodes, conventional monopolar straight electrodes and novel curved electrodes were used in the ablation of the bovine liver (n = 90). The ablated area, parallel axis, vertical axis and shape of different electrodes were compared. Then, 24 beagle dogs (10 months old, female) were used for in vivo experiments. Visual tumor targets deeply located in the portal vein were established, and ultrasound-guided liver ablation was performed with different electrodes. The ablation range, target coverage rate, percentage of normal tissue injury and damage to adjacent vessels were evaluated. The Kruskal–Wallis test and the Chi-squared test were used for statistical analysis.
Results
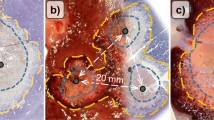
For the ex vivo study with a 3-cm electrode, the ablation area of the multi-tines expandable electrode group (7.14 ± 0.16 cm2) was significantly larger than that of the novel curved electrode group (5.01 ± 0.30 cm2, P < 0.001) and the monopolar straight electrode group (5.43 ± 0.15 cm2, P < 0.001). The results obtained with the 4-cm electrode in the three groups were in accordance with those of the 3-cm electrode. In vivo, the normal tissue damage area of the novel curved electrode group was smaller than that of the multi-tines expandable electrode group (1.10 ± 0.18 cm2 vs. 4.00 ± 0.18 cm2, P < 0.001). The target coverage rate of the novel curved electrode group was better than that of the monopolar straight electrode group (100% vs. 80.86 ± 1.68%, P < 0.001). The hematoxylin and eosin (H&E) and TUNEL staining results showed that the ablation necrosis area was adjacent to large vessels, but the vascular wall was not significantly damaged in the novel curved electrode group.
Conclusion
Our preliminary results showed that the novel curved RFA electrode with controllable direction could achieve accurate ablation for tumors behind large hepatic vessels, with a better target coverage rate and less damage to normal tissue, than conventional multi-tines expandable electrodes and monopolar straight electrodes.






Similar content being viewed by others
References
Ziemlewicz TJ, Wells SA, Lubner MG, Brace CL, Lee FT Jr, Hinshaw JL. Hepatic tumor ablation. Surg Clin North Am. 2016;96(2):315–39. https://doi.org/10.1016/j.suc.2015.12.006.
Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol. 2004;5(9):550–60. https://doi.org/10.1016/S1470-2045(04)01567-0.
Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10(4):338–47. https://doi.org/10.1634/theoncologist.6-1-14.
Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10(1):38–46. https://doi.org/10.1053/j.tvir.2007.08.006.
Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13(2):129–47. https://doi.org/10.1016/S0929-8266(01)00126-4.
McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW, Liu DM, Kee ST. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol. 2010;21(8 Suppl):S204–13. https://doi.org/10.1016/j.jvir.2009.11.025.
Ikeda K, Osaki Y, Nakanishi H, Nasu A, Kawamura Y, Jyoko K, Sano T, Sunagozaka H, Uchino K, Minami Y, Saito Y, Nagai K, Inokuchi R, Kokubu S, Kudo M. Recent progress in radiofrequency ablation therapy for hepatocellular carcinoma. Oncology. 2014;87(Suppl 1):73–7. https://doi.org/10.1159/000368148.
Ni Y, Mulier S, Miao Y, Michel L, Marchal G. A review of the general aspects of radiofrequency ablation. Abdom Imaging. 2005;30(4):381–400. https://doi.org/10.1007/s00261-004-0253-9.
Terraz S, Constantin C, Majno PE, Spahr L, Mentha G, Becker CD. Image-guided multipolar radiofrequency ablation of liver tumours: initial clinical results. Eur Radiol. 2007;17(9):2253e61. https://doi.org/10.1007/s00330-007-0626-x.
Lin SM, Lin CC, Chen WT, Chen YC, Hsu CW. Radiofrequency ablation for hepatocellular carcinoma: a prospective comparison of four radiofrequency devices. J Vasc Interv Radiol. 2007;18(9):1118–25. https://doi.org/10.1016/j.jvir.2007.06.010.
Fu JJ, Wang S, Yang W, Gong W, Jiang AN, Yan K, Chen MH. Protective and heat retention effects of thermo-sensitive basement membrane extract (matrigel) in hepatic radiofrequency ablation in an experimental animal study. Cardiovasc Intervent Radiol. 2017;40(7):1077–85. https://doi.org/10.1007/s00270-017-1617-1.
Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2(3):151–8. https://doi.org/10.3348/kjr.2001.2.3.151.
Lee JM, Han JK, Lee JY, Kim SH, Choi JY, Lee MW, Choi SH, Eo H, Choi BI. Hepatic radiofrequency ablation using multiple probes: ex vivo and in vivo comparative studies of monopolar versus multipolar modes. Korean J Radiol. 2006;7(2):106–17. https://doi.org/10.3348/kjr.2006.7.2.106.
Yoon JH, Lee JM, Han JK, Choi BI. Dual switching monopolar radiofrequency ablation using a separable clustered electrode: comparison with consecutive and switching monopolar modes in ex vivo bovine livers. Korean J Radiol. 2013;14(3):403–11. https://doi.org/10.3348/kjr.2013.14.3.403.
Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88(11):2452–63. https://doi.org/10.1002/1097-0142(20000601)88:11%3c2452:AID-CNCR5%3e3.0.CO;2-3.
Lee ES, Lee JM, Kim KW, Lee IJ, Han JK, Choi BI. Evaluation of the in vivo efficiency and safety of hepatic radiofrequency ablation using a 15-G Octopus® in pig liver. Korean J Radiol. 2013;14:194–201. https://doi.org/10.3348/kjr.2013.14.2.194.
Gao J, Wang SH, Ding XM, Sun WB, Li XL, Xin ZH, Ning CM, Guo SG. Radiofrequency ablation for single hepatocellular carcinoma 3 cm or less as first-line treatment. World J Gastroenterol. 2015;21(17):5287–94. https://doi.org/10.3748/wjg.v21.i17.5287.
Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67(2):336–47. https://doi.org/10.1016/j.ejrad.2007.07.007.
Lee YH, Hsu CY, Chu CW, Liu PH, Hsia CY, Huang YH, Su CW, Chiou YY, Lin HC, Huo TI. Radiofrequency ablation is better than surgical resection in patients with hepatocellular carcinoma within the Milan criteria and preserved liver function: a retrospective study using propensity score analyses. J Clin Gastroenterol. 2015;49(3):242–9. https://doi.org/10.1097/MCG.0000000000000133.
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–68. https://doi.org/10.1148/radiol.12111851.
Rhim H, Lim HK, Kim YS, Choi D, Lee WJ. Radiofrequency ablation of hepatic tumors: lessons learned from 3000 procedures. J Gastroenterol Hepatol. 2008;23(10):1492–500. https://doi.org/10.1111/j.1440-1746.2008.05550.x.
Appelbaum L, Sosna J, Pearson R, Perez S, Nissenbaum Y, Mertyna P, Libson E, Goldberg SN. Algorithm optimization for multitined radiofrequency ablation: comparative study in ex vivo and in vivo bovine liver. Radiology. 2010;254(2):430–40. https://doi.org/10.1148/radiol.09090207.
Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities–part II. J Vasc Interv Radiol. 2001;12(10):1135–48. https://doi.org/10.1016/S1051-0443(07)61670-4.
Stippel DL, Bangard C, Prenzel K, Yavuzyasar S, Fischer JH, Hölscher AH. Which parameters are needed for targeting a multitined radiofrequency device: an approach to a simple algorithm. Langenbecks Arch Surg. 2009;394(4):671–9. https://doi.org/10.1007/s00423-008-0306-6.
Cha J, Kim YS, Rhim H, Lim HK, Choi D, Lee MW. Radiofrequency ablation using a new type of internally cooled electrode with an adjustable active tip: an experimental study in ex vivo bovine and in vivo porcine livers. Eur J Radiol. 2011;77(3):516–21. https://doi.org/10.1016/j.ejrad.2009.09.011.
Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G. A novel “cooled-wet” electrode for radiofrequency ablation. Eur Radiol. 2000;10(5):852–4. https://doi.org/10.1007/s003300051018.
Furse A, Miller BJ, McCann C, Kachura JR, Jewett MA, Sherar MD. Radiofrequency coil for the creation of large ablations: ex vivo and in vivo testing. J Vasc Interv Radiol. 2012;23(11):1522–8. https://doi.org/10.1016/j.jvir.2012.08.015.
Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, Kim PN. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18(6):1624–9. https://doi.org/10.1245/s10434-011-1673-8.
Zhu K, Huang J, Lai L, Huang W, Cai M, Zhou J, Guo Y, Chen J. Medium or large hepatocellular carcinoma: sorafenib combined with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;288(1):300–7. https://doi.org/10.1148/radiol.2018172028.
Yuan H, Liu F, Li X, Guan Y, Wang M. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided radiofrequency ablation in the treatment of solitary large hepatocellular carcinoma. Radiol Med. 2018. https://doi.org/10.1007/s11547-018-0932-1.
Francica G, Meloni MF, Riccardi L, de Sio I, Terracciano F, Caturelli E, Iadevaia MD, Amoruso A, Roselli P, Chiang J, Scaglione M, Pompili M. Ablation treatment of primary and secondary liver tumors under contrast-enhanced ultrasound guidance in field practice of interventional ultrasound centers. A multicenter study. Eur J Radiol. 2018;105:96–101. https://doi.org/10.1016/j.ejrad.2018.05.030.
Noeren N, Nijkamp MW, Berendsen T, Govaert KM, van Kessel CS, Borel Rinkes IH, van Hillegersberg R. Multipolar radiofrequency ablation for colorectal liver metastases close to major hepatic vessels. Surgeon. 2015;13(2):77–82. https://doi.org/10.1016/j.surge.2013.11.013.
Haemmerich D, Wright AW, Mahvi DM, Lee FT Jr, Webster JG. Hepatic bipolar radiofrequency ablation creates coagulation zones close to blood vessels: a finite element study. Med Biol Eng Comput. 2003;41:317e23. https://doi.org/10.1007/BF02348437.
Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14(10):1267–74. https://doi.org/10.1097/01.RVI.0000092666.72261.6B.
Ahmed M, Liu Z, Afzal KS, Weeks D, Lobo SM, Kruskal JB, Lenkinski RE, Goldberg SN. Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology. 2004;230(3):761–7. https://doi.org/10.1148/radiol.2303021801.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81773286) and the Capital Characteristic Clinical Application Foundation (No. Z161100000516061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standard
All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee (Peking University, Cancer Hospital). All applicable institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, AN., Wang, S., Yang, W. et al. The Role of a Curved Electrode with Controllable Direction in the Radiofrequency Ablation of Liver Tumors Behind Large Vessels. Cardiovasc Intervent Radiol 42, 893–904 (2019). https://doi.org/10.1007/s00270-019-02182-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02182-0