Abstract
Purpose
To evaluate the efficacy and safety of the EGIS esophageal stent for treating malignant and benign esophageal strictures.
Materials and Methods
Data of 73 patients (mean age 63.0 ± 11.9 years; 66 males) with malignant esophageal stricture and 16 patients (mean age 63.7 ± 9.5 years; 13 males) with benign esophageal stricture who received the EGIS esophageal stent (S&G Biotech, Seongnam, Korea) between October 2010 and April 2016 were obtained from a prospectively maintained electronic database.
Results
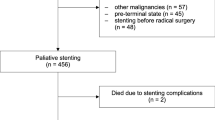
Technical and clinical success rates were 100% (89/89). Stent malfunction (i.e., tumor/tissue overgrowth, stent migration, and food impaction) occurred in 20.5% (15/73) and 37.5% (6/16) of patients with malignant and benign esophageal strictures, respectively. Stent migration occurred in five (6.8%) and four (25%) patients with malignant and benign esophageal strictures, respectively. The median follow-up durations in patients with malignant and benign esophageal strictures were 130 [interquartile range (IQR) 76–322] days and 486 (IQR 315–736) days, respectively. Recurrent dysphagia occurred in 14.1% (10/73) and 87.5% (14/16) of patients with malignant and benign esophageal strictures, respectively. The median recurrence-free durations in patients with malignant and benign esophageal strictures were 126 (IQR 69–259) days and 100 (IQR 40–182) days, respectively.
Conclusion
The EGIS esophageal stent appears to be effective for malignant esophageal strictures, with relatively low rate of stent migration, whereas, for benign esophageal strictures, it seems to be associated with a high rate of recurrent dysphagia, mainly due to stent migration.





Similar content being viewed by others
References
Spaander MC, Baron TH, Siersema PD, Fuccio L, Schumacher B, Escorsell A, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2016;48(10):939–48.
Song HY, Choi KC, Cho BH, Ahn DS, Kim KS. Esophagogastric neoplasms: palliation with a modified gianturco stent. Radiology. 1991;180(2):349–54.
Na HK, Song HY, Kim JH, Park JH, Kang MK, Lee J, et al. How to design the optimal self-expandable oesophageal metallic stents: 22 years of experience in 645 patients with malignant strictures. Eur Radiol. 2013;23(3):786–96.
Kim JH, Song HY, Shin JH, Jung HY, Kim SB, Kim JH, et al. Membrane degradation of covered stents in the upper gastrointestinal tract: frequency and clinical significance. J Vasc Interv Radiol. 2008;19(2 Pt 1):220–4.
Park JH, Song HY, Kim JH, Jung HY, Kim JH, Kim SB, et al. Polytetrafluoroethylene-covered retrievable expandable nitinol stents for malignant esophageal obstructions: factors influencing the outcome of 270 patients. AJR Am J Roentgenol. 2012;199(6):1380–6.
Park JH, Song HY, Shin JH, Cho YC, Kim JH, Kim SH, et al. Migration of retrievable expandable metallic stents inserted for malignant esophageal strictures: incidence, management, and prognostic factors in 332 patients. AJR Am J Roentgenol. 2015;204(5):1109–14.
Kim JH, Song HY, Choi EK, Kim KR, Shin JH, Lim JO. Temporary metallic stent placement in the treatment of refractory benign esophageal strictures: results and factors associated with outcome in 55 patients. Eur Radiol. 2009;19(2):384–90.
Kim JH, Song HY, Shin JH, Lim JO, Kim KR, Kwon JH, et al. Anastomotic recurrence of gastric cancer after total gastrectomy with esophagojejunostomy: palliation with covered expandable metallic stents. J Vasc Interv Radiol. 2007;18(8):964–9.
Song HY, Jung HY, Park SI, Kim SB, Lee DH, Kang SG, et al. Covered retrievable expandable nitinol stents in patients with benign esophageal strictures: initial experience. Radiology. 2000;217(2):551–7.
Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–202.
Sharma P, Kozarek R. Practice Parameters Committee of American College of G. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105(2):258–73 quiz 74.
Uitdehaag MJ, van Hooft JE, Verschuur EM, Repici A, Steyerberg EW, Fockens P, et al. A fully-covered stent (Alimaxx-E) for the palliation of malignant dysphagia: a prospective follow-up study. Gastrointest Endosc. 2009;70(6):1082–9.
Uitdehaag MJ, Siersema PD, Spaander MC, Vleggaar FP, Verschuur EM, Steyerberg EW, et al. A new fully covered stent with antimigration properties for the palliation of malignant dysphagia: a prospective cohort study. Gastrointest Endosc. 2010;71(3):600–5.
Kim MD, Park SB, Kang DH, Lee JH, Choi CW, Kim HW, et al. Double layered self-expanding metal stents for malignant esophageal obstruction, especially across the gastroesophageal junction. World J Gastroenterol. 2012;18(28):3732–7.
Park JG, Jung GS, Oh KS, Park SJ. Double-layered PTFE-covered nitinol stents: experience in 32 patients with malignant esophageal strictures. Cardiovasc Intervent Radiol. 2010;33(4):772–9.
Walter D, van den Berg MW, van Hooft JE, Boot H, Scheffer RC, Vleggaar FP, et al. A new fully covered metal stent with anti-migration features for the treatment of malignant dysphagia. Endoscopy. 2014;46(12):1101–5.
Repici A, Jovani M, Hassan C, Solito B, Di Mitri R, Buffoli F, et al. Management of inoperable malignant oesophageal strictures with fully covered WallFlex((R)) stent: a multicentre prospective study. Dig Liver Dis. 2014;46(12):1093–8.
Talreja JP, Eloubeidi MA, Sauer BG, Al-Awabdy BS, Lopes T, Kahaleh M, et al. Fully covered removable nitinol self-expandable metal stents (SEMS) in malignant strictures of the esophagus: a multicenter analysis. Surg Endosc. 2012;26(6):1664–9.
Canena JM, Liberato MJ, Rio-Tinto RA, Pinto-Marques PM, Romao CM, Coutinho AV, et al. A comparison of the temporary placement of 3 different self-expanding stents for the treatment of refractory benign esophageal strictures: a prospective multicentre study. BMC Gastroenterol. 2012;12:70.
Chaput U, Heresbach D, Audureau E, Vanbiervliet G, Gaudric M, Bichard P, et al. Comparison of a standard fully covered stent with a super-thick silicone-covered stent for the treatment of refractory esophageal benign strictures: a prospective multicenter study. United Europ Gastroenterol J. 2013;1(2):93–102.
Dan DT, Gannavarapu B, Lee JG, Chang K, Muthusamy VR. Removable esophageal stents have poor efficacy for the treatment of refractory benign esophageal strictures (RBES). Dis Esophagus. 2014;27(6):511–7.
Hindy P, Hong J, Lam-Tsai Y, Gress F. A comprehensive review of esophageal stents. Gastroenterol Hepatology. 2012;8(8):526–34.
Na HK, Song HY, Yeo HJ, Park JH, Kim JH, Park H, et al. Retrospective comparison of internally and externally covered retrievable stent placement for patients with benign urethral strictures caused by traumatic injury. AJR Am J Roentgenol. 2012;198(1):W55–61.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant Number: HI15C0484 to H.Y.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
This retrospective study was approved by our institutional review board, and the requirement for written informed consent was waived.
Rights and permissions
About this article
Cite this article
Kim, K.Y., Tsauo, J., Song, HY. et al. Evaluation of a New Esophageal Stent for the Treatment of Malignant and Benign Esophageal Strictures. Cardiovasc Intervent Radiol 40, 1576–1585 (2017). https://doi.org/10.1007/s00270-017-1677-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1677-2