Abstract
Purpose
We retrospectively evaluated the outcomes of lung cancer patients presenting with ground-glass opacity (GGO) who received radiofrequency ablation (RFA).
Methods
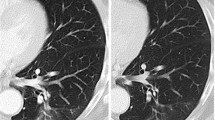
Sixteen patients (5 men and 11 women; mean age, 72.6 years) with 17 lung cancer lesions showing GGO (mean long axis diameter, 1.6 cm) underwent a total of 20 percutaneous computed tomography (CT) fluoroscopy-guided RFA sessions, including three repeated sessions for local progression. Lung cancer with GGO was defined as a histologically confirmed malignant pulmonary lesion with a GGO component accounting for >50 % of the lesion on high-resolution CT. Procedure outcomes were evaluated.
Results
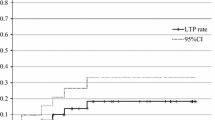
There were no major complications. Pneumothorax occurred in 15 of 20 treatment sessions: 14 were asymptomatic, and 1 required chest tube placement but resolved satisfactorily within 48 h. Minor pulmonary hemorrhage occurred in two and mild pneumonitis in one. The median tumor follow-up period was 61.5 (range 6.1–96.6) months. The effectiveness rates of the primary and secondary techniques were 100 and 100 % at 1 year, 93.3 and 100 % at 2 years, and 78.3 and 92.3 % at 3 years, respectively. The median patient follow-up period was 65.6 (range 6.1–96.6) months. One patient died owing to recurrent other cancer 11.7 months after RFA, whereas the other 15 remained alive. Overall survival and disease-specific survival rates were 93.3 and 100 % at 1 year and 93.3 and 100 % at 5 years, respectively.
Conclusions
RFA for lung cancer with GGO was safe and effective, and resulted in promising survival rates.


Similar content being viewed by others
References
Godoy MC, Naidich DP (2009) Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 253(3):606–622 PMID: 19952025
Hiraki T, Sakurai J, Tsuda T et al (2006) Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a preliminary review of 342 tumors. Cancer 107(12):2873–2880 PMID: 17096433
Okuma T, Matsuoka T, Yamamoto A et al (2010) Determinants of local progression after computed tomography-guided percutaneous radiofrequency ablation for unresectable lung tumors: 9-year experience in a single institution. Cardiovasc Intervent Radiol 33(4):787–793 PMID: 19967367
de Baère T, Palussière J, Aupérin A et al (2006) Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 240(2):587–596 PMID: 16864679
Lencioni R, Crocetti L, Cioni R et al (2008) Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 9(7):621–628 PMID: 18565793
Kim TJ, Lee JH, Lee CT et al (2008) Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 190(1):234–239 PMID: 18094317
Hur J, Lee HJ, Nam JE et al (2009) Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 192(3):629–634 PMID: 19234257
Hiraki T, Gobara H, Mimura H et al (2011) Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 142(1):24–30 PMID: 21529847
Hiraki T, Gobara H, Mimura H et al (2011) Radiofrequency ablation of lung cancer at Okayama University Hospital: a review of 10 years of experience. Acta Med Okayama 65(5):287–297 PMID: 22037265
Yamamoto A, Nakamura K, Matsuoka T et al (2005) Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. AJR Am J Roentgenol 185(5):1299–1306 PMID: 16247153
Abtin FG, Eradat J, Gutierrez AJ, Lee C, Fishbein MC, Suh RD (2012) Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 32(4):947–969 PMID: 22786987
Hiraki T, Gobara H, Mimura H et al (2010) Does tumor type affect local control by radiofrequency ablation in the lungs? Eur J Radiol 74(1):136–141 PMID: 19231125
Goldberg SN, Grassi CJ, Cardella JF et al (2009) Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 20(7 Suppl):S377–S390 PMID: 19560026
Noguchi M, Morikawa A, Kawasaki M et al (1995) Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 75(12):2844–2852 PMID: 7773933
Kondo T, Yamada K, Noda K, Nakayama H, Kameda Y (2002) Radiologic-prognostic correlation in patients with small pulmonary adenocarcinomas. Lung Cancer 36(1):49–57 PMID: 11891033
Asamura H, Suzuki K, Watanabe S, Matsuno Y, Maeshima A, Tsuchiya R (2003) A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg 76(4):1016–1022 PMID: 14529977
Nakao M, Yoshida J, Goto K et al (2012) Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 7(10):1563–1566 PMID: 22878750
Hamamoto Y, Kataoka M, Yamashita M et al (2012) Factors affecting the local control of stereotactic body radiotherapy for lung tumors including primary lung cancer and metastatic lung tumors. Jpn J Radiol 30(5):430–434 PMID: 22450903
Simon CJ, Dupuy DE, DiPetrillo TA et al (2007) Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 243(1):268–275 PMID: 17392258
Ambrogi MC, Fanucchi O, Cioni R et al (2011) Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol 6(12):2044–2051 PMID: 22052222
Yasui K, Kanazawa S, Sano Y et al (2004) Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology 231(3):850–857 PMID: 15105453
Anderson EM, Lees WR, Gillams AR (2009) Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol 32(3):478–483 PMID: 19127381
Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY (2010) The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol 195(3):758–765 PMID: 20729457
Kim KW, Lee JM, Klotz E et al (2011) Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol 196(5):W565–W572 PMID: 21512046
Hasegawa M, Sone S, Takashima S et al (2000) Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 73(876):1252–1259 PMID: 11205667
Kim TJ, Park CM, Goo JM, Lee KW (2012) Is there a role for FDG PET in the management of lung cancer manifesting predominantly as ground-glass opacity? AJR Am J Roentgenol 198(1):83–88 PMID: 22194482
Kashima M, Yamakado K, Takaki H et al (2011) Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center’s experiences. AJR Am J Roentgenol 197(4):W576–W580 PMID: 21940529
Hiraki T, Mimura H, Gobara H et al (2008) Repeat radiofrequency ablation for local progression of lung tumors: does it have a role in local tumor control? J Vasc Interv Radiol 19(5):706–711 PMID: 18440459
Conflict of interest
Toshihiro Iguchi, Takao Hiraki, Hideo Gobara, Hiroyasu Fujiwara, Yusuke Matsui, Junichi Soh, Shinichi Toyooka, Katsuyuki Kiura, and Susumu Kanazawa have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iguchi, T., Hiraki, T., Gobara, H. et al. Percutaneous Radiofrequency Ablation of Lung Cancer Presenting as Ground-Glass Opacity. Cardiovasc Intervent Radiol 38, 409–415 (2015). https://doi.org/10.1007/s00270-014-0926-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0926-x