Abstract
Background
The purpose of this study was to find whether the use of hyaluronidase reduces capsule formation.
Methods
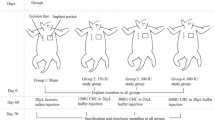
Ten New Zealand White rabbits were used. Eight pockets were created using an aseptic technique, four on the right side and four on the left side, along the vertebral column of every animal. One piece of silicone from a silicone block was inserted inside every pocket. The dimensions of each piece were 3.5 × 2 × 1.5 cm. In every pocket on the right side of each animal we placed 0.5 ml of hyaluronidase solution. The animals were sacrificed 5 months postoperatively. Capsule formation in the each side of the animals was compared.
Results
Two rabbits presented infection in two pockets and were excluded from the study. There was a statistically significant difference between groups concerning the capsule thickness variable using parametric (P = 0.003) and nonparametric (P = 0.001) analysis [capsule thickness on the right side: 256.46 ± 114.88 (mean ± SD) and on the left side: 369.10 ± 147.81 (mean ± SD); capsule thickness on the right side: 235.69 (104.72) [median (IQR)] and on the left side: 332.12 (188.68) median (IQR)].
Conclusion
The use of hyaluronidase may reduce capsule formation around implants.




Similar content being viewed by others
References
Ajmal N, Riordan CL, Cardwell N, Nanney LB, Shack RB (2003) The effectiveness of sodium 2-mercaptoethane sulfonate (Mesna) in reducing capsular formation around implants in a rabbit model. Plast Reconstr Surg 112:1455–1461 discussion 1462–1463
Burckhardt BR, Dempsey PD, Schnur PL, Tofield JJ (1986) Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg 77:919–930
Dobke MK, Svahn JK, Vastine VL, Landon BN, Stein PC, Parsons CL (1995) Characterization of microbial presence at the surface of silicone mammary implants. Ann Plast Surg 34:563–569 discussion 570–571
Dowden RV (1994) Periprosthetic bacteria and the breast of implant patient with systemic symptoms. Plast Reconstr Surg 94:300–305
Netscher DT, Weizer G, Wigoda P, Walker LE, Thornby J, Bowen D (1995) Clinical relevance of positive breast peri-prosthetic cultures without overt infection. Plast Reconstr Surg 96:1125–1129
Virden CP, Dobke MK, Stein P, Parsons CL, Frank DH (1992) Subclinical infection of silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast Surg 16:173–179
Smahel J (1977) Histology of capsule causing constrictive fibrosis around breast implants. Br J Plast Surg 30:324–329
Wilflingsedar P, Propst A, Micuz G (1974) Constrictive fibrosis following silicone implants in mammary augmentation. Chir Plast (Berl) 2:215–229
Williams C, Aston S, Rees TD (1975) The effect of haematoma on the thickness of pseudosheaths around silicone implants. Plast Reconstr Surg 56:194–198
Matsuoka M, Ono G (1963) Effect of hyaluronidase on blood coagulation. Sogo Igaku 20:375–385
Cachay-Velasquez H, Ale A (1990) Lateral approach in mammary implants. Ann Plast Surg 25:258–262
Bern S, Burd A, May JW Jr (1992) The biophysical and histologic properties of capsules formed by smooth and textured silicone implants in the rabbit. Plast Reconstr Surg 89:1037–1042
Rudolph R, Abraham J (1980) Tissue effects of new silicone mammary-type implants in rabbits. Ann Plast Surg 4:14–20
Darouiche RO, Meade R, Mansouri MD, Netscher DT (2002) In vivo efficacy of antimicrobe-impregnated saline-filled silicone implants. Plast Reconstr Surg 109:1352–1357
Buckhardt BR, Eades E (1995) The effect of biocell texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg 96:1317–1325
Lemperle G, Exner K (1993) Effect of cortisone on capsular contracture in double-lumen breast implants: ten years’ experience. Aesthetic Plast Surg 17:317–323
O’Neal RM, Argenta LC (1982) Late side effects related to inflatable breast prostheses containing soluble steroids. Plast Reconstr Surg 69:641–645
The electronic Medicines Compendium (eMC) (2008) Hyalase 1500 I.U. Powder for solution for injection/infusion. http://www.medicines.org.uk/emc/document.aspx?documentid=7315&docType=SPC (assessed 24 May 2010)
Frost GI (2007) Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv 4:427–440
Calder IG, Smith VH (1986) Hyaluronidase and sodium hyaluronate in cataract surgery. Br J Opthalmol 70:418–420
Farr C, Menzel J, Seeberger J, Schweigle B (1997) Clinical pharmacology and possible applications of hyaluronidase with reference to Hylase “Dessau”. Wien Med Wochenschr 147:347–355
Born T (2006) Hyaluronic acids. Clin Plast Surg 33:525–538
Kempeneers A, Dralands L, Ceuppens J (1992) Hyaluronidase induced orbital pseudotumor as complication of retrobulbar anesthesia. Bull Soc Belge Opthalmol 243:159–166
Quaglini V, Mantero S, Villa T (2005) Mechanical properties of breast periprosthetic capsules and the correlation to capsule contracture. J Appl Biomater Biomech 3:184–191
Cherup LL, Antaki JF, Liang MD, Hamas RS (1989) Measurement of capsular contracture: the conventional breast implant and the Pittsburgh implant. Plast Reconstr Surg 84:893–901
Acknowledgments
We thank Paraskevi Mproumi and Platon Trigkatzis for their help in performing the experiment.
Disclosures
The authors have no commercial interest in the subject of study and no financial or material support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spyropoulou, GA., Papalois, A., Batistatou, A. et al. Can the Use of Hyaluronidase Reduce Capsule Formation?. Aesth Plast Surg 35, 782–788 (2011). https://doi.org/10.1007/s00266-011-9687-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-011-9687-y