Abstract
After more than 25 years of research and development, in October 2006 ArteFill® became the first and only permanent injectable wrinkle filler to receive FDA approval. ArteFill is a third-generation polymeric microsphere-based filler, following its predecessor Artecoll®, which was marketed outside the United States between 1994 and 2006. ArteFill is approved for the correction of nasolabial folds and has been used in over 15,000 patients since its U.S. market introduction in February 2007. No serious side effects have been reported to date according to the FDA’s MAUDE reporting database. ArteFill consists of polymethylmethacrylate (PMMA) microspheres (20% by volume), 30–50 μm in diameter, suspended in 3.5% bovine collagen solution (80% by volume) and 0.3% lidocaine. The collagen carrier is absorbed within 1 month after injection and completely replaced by the patient’s own connective tissue within 3 months. Each cc of ArteFill contains approximately six million microspheres and histological studies have shown that long-term wrinkle correction consists of 80% of the patient’s own connective tissue and 20% microspheres. The standard injection technique is subdermal tunneling that delivers a strand of ArteFill at the dermal–subdermal junction. This strand beneath a wrinkle or fold acts like a support structure that protects against further wrinkling and allows the diminished thickness of the dermis to recover to its original thickness.
Similar content being viewed by others
The first commercially successful, FDA-approved injectable filler for soft tissue augmentation was a solution of soluble collagen [1, 2] marketed by Collagen Corporation in 1983. Until then, the only widely used “off-the-shelf” injectable filler had been liquid dimethylpolysiloxane (silicone). Complications from liquid silicone (LIS), however, were often disfiguring and challenging to correct.
Collagen injections, on the other hand, were never held out as permanent corrective devices for the treatment of soft tissue defects, whether scar depressions, atrophy, or wrinkle lines. The corollary mantras “temporary solution, but temporary problems” (if an adverse event should occur), and “permanent solution, permanent problems” were associated with collagen and silicone, respectively. However, although both patients and physicians accepted the inconvenience and repeated expense of short-term fillers, they have long sought a dermal filler substance that promised long-lasting results with extremely low incidences of complications and adverse events. With the development of ArteFill® (Suneva Medical, San Diego, CA), a viscous liquid of polymethylmethacrylate (PMMA) microspheres suspended in solubilized bovine collagen, and its approval by the FDA in 2006, a long-lasting subdermal filler is now lawfully available.
Development History
ArteFill Precursors
The PMMA/collagen predecessor to ArteFill was first developed by the senior author in Germany more than 20 years ago, and his persistent efforts led to the current (third generation) product, ArteFill [3]. The combination of two widely used and proven biocompatible materials, bovine collagen (sutures, hemostatic agents, implants) and PMMA (orthopedic bone cement, craniectomy plates), satisfies biocompatibility issues. The microscopically small particles of PMMA in the bovine collagen carrier are enveloped by autologous collagen as the byproduct of natural connective tissue turnover, leaving a pliable, and permanent, tissue residual.
After seeking the optimal collagen/PMMA ratios in laboratory animals, trials on humans were initiated. The first-generation product, called Arteplast®, as well as its successor, Artecoll®, proved efficacious, although adverse events did emerge [3–5]. Most of these adverse events were firm nodularities at injection sites, occasionally with an associated inflammatory response. In several instances, surgical removal of the implant was required.
Further investigation led to the conclusion that there is a specific threshold of PMMA microsphere size that is critical to avoid phagocytosis by macrophages and giant cell formation with resulting granulomatous inflammation. Associated observations suggested that small PMMA microspheres, less than 20 μm in diameter, engendered a foreign body response [6]. ArteFill embodies the lessons learned from both the Arteplast and Artecoll experiences.
Biocompatibility
The key to ArteFill’s biocompatibility and safety, as documented in animal experiments [7], is the extremely uniform, round and smooth PMMA microspheres [3], and especially the absence of particles less than 20 μm in diameter (Fig. 1). The novel purification process established for the production of the final product explains the absence of documented granulomas with ArteFill in over 15,000 patients. These observations differ from the granuloma rates reported after the injection of first-generation Arteplast and second-generation Artecoll [6]. In those cases, host cellular reaction was histologically attributed to PMMA impurities and PMMA particles smaller than 20 μm that could be phagocytized [7]. In the earlier processing of the PMMA microspheres, the small particles appeared to adhere to the larger microspheres during the sieving step, probably due to electrostatic charges.
The smooth surface morphology of ArteFill’s PMMA microspheres also appears to mitigate an inflammatory response. Microscopically, macrophages and foreign body giant cells, also called “frustrated macrophages,” can be detected around particles with an irregular surface [8]. This may explain the rather high rate of granulomas after injection of Dermalive®, whose particles are characterized by an irregular, rugged surface [6]. It has also been observed that spiculated and small, irregular particulate materials such as polyurethane foam and the silicone particles on the surface of textured breast implants often elicit a chronic granulomatous tissue reaction [6].
Histology
ArteFill, as with implantation of any particulate material in humans, invariably elicits an initial foreign body reaction. As with normal wound healing, the initial event consists of a tissue-material interaction whereby serum proteins (fibronectin and fibrinogen) are deposited at the microsphere surface. The next event is the invasion of neutrophils and monocytes, which release their granular components and rapidly differentiate into macrophages. They attach to the microspheres and form a monocellular layer over all smooth surfaces. When rough surfaces are present, even on larger particles, macrophages may morph into giant cells in a frustrated attempt to phagocytize the offending foreign body.
The third reaction to foreign material is the formation of “granulation tissue,” composed of macrophages, fibroblasts, capillaries, and collagen that fill the interstitial spaces between the microspheres. The bovine collagen [1, 2] appears to maintain the separation between the microspheres and facilitates tissue ingrowth (Fig. 2). Without ArteFill’s collagen component the microspheres would clump together—a phenomenon observed in other filler formulations using hyaluronic acid-based or methylcellulose carriers [9].
Approximately 4 weeks after implantation the ArteFill implant consists of 20% inert microspheres and 80% granulation tissue. This ratio may vary at 4 weeks depending on the volume of the material implanted (Fig. 3). Subsequently, over time the connective tissue matures through a natural process similar to scar formation and the interstices are filled with fibroblasts and autologous collagen fibers.
Histology at 3 months demonstrates that all of the PMMA microspheres are completely encapsulated and are surrounded by fibroblasts and collagen fibers. Macrophages are rare and capillary in-growth is evident (Fig. 4).
Human histology after 10 years revealed strong bands of mature collagen fibers with fully intact capillary vasculature surrounding intact PMMA microspheres (which were dissolved by alcohol during histology processing) (Fig. 5). In essence, the ArteFill injection serves as a scaffold to promote a “living implant.” The PMMA components of ArteFill become fully integrated into the connective tissue, whether dermis or subdermal spaces. As in normal tissue with sufficient blood supply, there appears to be constant turnover of cells, including fibroblasts and macrophages.
Mechanism of Action
Unlike any other injectable filler, ArteFill appears to stimulate the patient’s own collagen production, which then permanently envelopes the PMMA microspheres [10]. A key advantage of the collagen carrier appears to be its viscosity [11], which keeps the microspheres evenly distributed and facilitates tissue ingrowth into the interstices. The residual, inert, nonmetabolizable PMMA remains as a scaffold to engender permanent autologous collagen replacement of the injected bovine collagen carrier. Other biologic filler materials in commercial use for wrinkle treatment and soft tissue augmentation are completely metabolized within 6 months to 1 year [12].
A sustained tissue augmentation effect appears achievable only with a nonabsorbable synthetic component to the filler (Fig. 6) [13, 14]. ArteFill’s 30–50-μm-diameter PMMA microspheres (Fig. 1) seem to be the ideal size for dermal injections—large enough to escape phagocytosis [7] yet small enough to be smoothly delivered through a fine 26G or 30G needle.
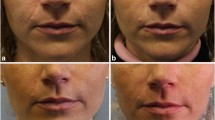
Blinded observer ratings according to the wrinkle assessment scale [18]. The same effect of ArteFill is kept 5 years after injection as after 6 months
There are other specific advantages to microspheres in this size range. The smaller the microspheres (to the threshold of phagocytosis), the larger their combined surface area in a given volume and the greater the total amount of new collagen deposition. Microspheres with a diameter of 100 μm, for example, promote the ingrowth of only about 56% connective tissue, whereas microspheres with a mean diameter of 40 μm promote the ingrowth of about 80% connective tissue [7]. The PMMA/collagen ratio and the 30–50 μm PMMA microsphere sizing for ArteFill are direct applications of these observations.
Two key features appear to prevent dissipation of ArteFill into the surrounding fine network of collagen fibers of the deep dermal and subdermal layers following injection: (1) The bovine collagen carrier acts as a glue, embedding the microspheres and preventing their clumping and allowing for new tissue ingrowth (Fig. 2). Thus, the implant becomes part of the patient’s own soft tissue, maintaining its integrity over years and decades. (2) The 30–50 μm microsphere size prevents both phagocytosis with removal and also limits the PMMA particles from entering the interstices of deep dermal fibers, which have a diameter of 10–15 μm [9], where muscle action might disperse them.
In wrinkle treatment, the resulting structural support of ArteFill’s six million microspheres per milliliter prevents further wrinkling and folding. This allows the diminished thickness of the corium to recover (Fig. 7). This wrinkle recovery process appears similar to the well-known phenomenon after facial palsy in older patients and in stroke victims (or repeat Botox® patients), whose facial wrinkles and furrows on the paralyzed side completely disappear over time simply as a result of the lack of movement.
The first ArteFill strand provides a soft “splint” beneath the wrinkle. A second “splint” after 1–3 months causes the diminished thickness of the dermis in a crease to recover its former thickness ([3] with permission from Elsevier)
Material and Methods
ArteFill Packaging
Each box of ArteFill contains 0.8 cc and 0.4 cc syringes. The syringe can be equipped with a double stopper for use in a Metered Dose Delivery (MDD) device that delivers precise and consistent microdroplets. The PMMA microspheres are suspended in a carrier of solubilized low antigenic bovine collagen 3.5% solution. The carrier collagen contains the local anesthetic lidocaine (0.3%) for injection comfort. The clear collagen solution allows the user to detect any phase separation between solid microspheres and viscous collagen. If phase separation is detected, the material should not be used. Refrigeration of ArteFill is recommended. The product’s shelf life is currently 12 months.
Allergy Testing
Bovine collagen allergy testing is required to minimize the risk of hypersensitivity reactions, especially in patients who are being treated with a bovine collagen product for the first time. An intradermal collagen test injection in the volar forearm 4 weeks prior to the planned ArteFill implantation is standard. Reports of allergic reactions to ArteFill are extremely rare. A recent ArteFill skin test study involving 1,000 patients revealed only two positive test results with elevated antibodies. This low allergy rate (0.2%) to ArteFill’s bovine collagen carrier, compared to a greater than 3% reactivity to the crosslinked bovine collagen contained in Zyderm®/Zyplast®, may eventually allow removal of FDA’s skin test requirement for ArteFill.
Although ArteFill collagen gel contains 0.3% lidocaine, field block local anesthetics may be used in very sensitive patients. Other useful techniques include a topical anesthetic spray (PainEase®) or a 5% lidocaine cream [11] or others (EMLA®, Betacaine®, Topicaine®) applied 30 min before the procedure.
At the beginning of the procedure, the physician should ensure that the needle is not blocked by gently squeezing a tiny drop of ArteFill out of its tip. Due to the microsphere content, the viscosity of ArteFill is about three times higher than that of collagen or hyaluronic acid and requires a bit more force for extrusion. Extrusion forces are 32.7 N for ArteFill compared to 11.2 N for Zyderm collagen.
Injection Technique
Tunneling Technique
Locating the correct plane for the injection of ArteFill permanent filler is of utmost importance. The thickness of the facial dermis varies between 0.4 mm in lids and 1.2 mm in the forehead and cheeks (Fig. 8). In a deep crease the thickness of the dermis may be reduced to only one-third of its normal thickness [3]. The outer diameter of a 26G needle is 0.45 mm and can be used as a depth gauge to estimate the thickness of the dermis. The facial dermis is only about twice as thick as a 26G needle (Fig. 9).
The thickness of the dermis varies greatly in different areas of the face (from [3])
The “tunneling technique”: Note the relationship of dermal thickness in a wrinkle and the diameter of a 26G needle—both are around 0.4 mm. The ArteFill strand is delivered while withdrawing the needle ([3] with permission from Elsevier)
With fine blunt microneedles of 26G one cannot achieve longitudinal penetration intradermally. Therefore, with this type needle it is easy to establish the correct plane for injection by manipulating the cannula tip along the dermis from beneath. This technique minimizes trauma and prevents bruising and intradermal ridges, especially in nasolabial folds. It requires only a single cannula penetration through a tiny stab incision [15].
For deep dermal injections in patients with facial lipodystrophy syndrome (LDS), blunt needles of 23G or larger will facilitate nudging venules and arterioles aside and prevent bruising, intravascular deposition of ArteFill, and potential embolism [15, 16].
The following specific steps for ArteFill injections have yielded consistently good results:
-
Prior to inserting the needle in the dermis, one may stretch the skin tightly to create a firm surface. The needle is inserted into the dermis at an approximately 10° angle, parallel to the length of the wrinkle or fold.
-
While maintaining constant thumb pressure on the plunger, insert the needle (with bevel either up or down) into the skin at the dermal/subdermal interface along the line of the wrinkle. ArteFill is implanted as the needle is withdrawn along the course of the wrinkle by placing a continuous strand of material under consistent pressure (resistance will be noted) into the junction of the reticular dermis and subcutaneous fat (Fig. 9).
-
If the needle placement is too superficial, the gray of the needle will be visible through the skin (Fig. 10) and tissue blanching will be observed upon injection. This indicates improper needle placement. The needle should be withdrawn and reinserted one needle diameter deeper. Blanching is a sign that the papillary plexus is compressed. If blanching occurs, distribute the implant into the surrounding dermis using fingernail massage.
-
If needle placement is too deep, the needle will be felt to “pop” the subcutaneous fat and no resistance will be felt when injecting the ArteFill (Fig. 11).
-
If the needle is in the correct plane, i.e., the junction of the dermis and subdermal fat, it will be possible to pull the dermis superficially causing a ridge or push the dermis deep causing a depression or groove (Fig. 12).
-
An even, continuous strand should be delivered while withdrawing the needle (Fig. 9). Using pressure on the plunger during the forward movement of the needle, a protruding droplet of ArteFill may create a “blunt needle tip” and preserve some of the capillaries in its way, e.g., it may prevent bruising.
-
Immediately after implant placement, the injector should palpate the implant by gently applying pressure and eventually evenly massage with the fingernail to facilitate uniform distribution. Overly vigorous massage may spread ArteFill deeper into the fatty tissue where it does not achieve the desired effect, and massage may cause unwanted swelling and bruising.
In the nasolabial fold one should stay approximately 1 mm medial to the crease to prevent lateral dislocation of the ArteFill implant by facial movement during the first few days following the injection. The nasolabial fold can be divided and treated as three regions: the lower, middle, and upper (subnasal triangle). Injection should begin in the upper portion of the fold because of consequent anesthesia of the lower parts of the nasolabial fold. In the upper region the needle is fanned to eventually fill a deep subnasal triangle. The needle is inserted and advanced forward as far as possible. While withdrawing (pulling back) the needle, constant even pressure is applied on the plunger, depositing and “anchoring” a uniform amount of ArteFill as one proceeds (Fig. 9). Resistance should be felt during injection. The wrinkle should be lifted and improved by the end of the injection.
With the exception of the soft vermilion, overcorrection is difficult if ArteFill is deposited in the correct plane, i.e., at the junction of the reticular dermis and subcutaneous fat. The density of the reticular dermis prevents intrusion of the material in excessive quantities.
The depth of needle placement and the quantity of ArteFill used to correct a wrinkle varies depending on the patient’s skin thickness and facial anatomy. If the patient wants an optimal result, a hyaluronic acid product can be injected into the reticular dermis above the ArteFill “splint” (Fig. 13). If the patient desires further correction of the wrinkle, the same procedure may need to be repeated until a satisfactory result is obtained (Fig. 7).
An optimal immediate result can be achieved by subdermal injections of ArteFill (1.) and intradermal injections of hyaluronic acid (2.) on top of it. ([3] with permission from Elsevier)
Serial Puncture Technique
Some injectors are skilled using the serial puncture technique developed for Zyderm/Zyplast [17]. In experienced hands it may be as effective as threading. Proponents maintain that it causes less bruising. An anesthetic cream 30 min before injection [11] may relieve the added discomfort from multiple needle punctures.
Precautions and Aftercare
-
The appearance of blanching indicates that the injection occurred intradermally and therefore is too shallow.
-
The implanted area should not be massaged because this may adversely distribute the ArteFill support “splint” and increase swelling and bruising. In contrast to temporary volume enhancers, ArteFill must not be spread in the tissue but remain inside the tunnel in which it was injected.
-
The implants should be palpated for even distribution. In case of lumpiness, pressure is applied by squeezing the nasolabial fold between thumb and index finger.
-
The treated fold should be taped with a 1-in. transparent tape (Transpore® or Blenderm®) for 3 days. The purpose of the tape is to remind the patient not to smile until the subdermal implant strands are fixated. ArteFill can be dislodged from the deep dermal implantation site into deeper layers through pronounced facial muscle movement during the first 3 days, diminishing the expected result. To prevent this, immobilization using tape is important during the first 3 days.
-
Patients should be advised that there is some swelling for the first 12–24 h. There is no indication for ice packs to reduce swelling or bruising. Some edema of the implant site is expected as a physiological necessity to allow macrophages and fibroblasts to invade the treatment area. Prevention of smiling for 3 days is more important! Any areas of light pink discoloration along the injection sites (2–5 days) can be covered with makeup.
Progressive Enhancement
In most cases touch-up treatments can be administered after 1–3 months. European experience with Artecoll has shown that approximately 50% of patients will require a second treatment to achieve full correction.
-
When performing a touch-up procedure, the original strand of implanted ArteFill can often be palpated with the needle tip to determine location.
-
The needle is inserted above the first implant and below the dermal crease in the deep dermis.
-
New strands of ArteFill should be layered on top of the original injected material. The new strands should not be affected by facial movement because they obtain support from the initial implant (Fig. 13).
The injected strands create a support structure (splint) for the wrinkle from beneath and prevent further wrinkling. In about 50 % of the patients, the original thickness of the dermis recovers by itself within approximately 3 months (Fig. 7).
Implant Volume
Grades I and II wrinkles [18] will require approximately one strand of ArteFill (~0.2 cc). Grades III–V wrinkles may need two to four strands (0.8 cc). Experience has shown that overcorrection with ArteFill is almost impossible. In the deep dermis beneath a wrinkle there is limited space available that can be filled with ArteFill. In the right plane, overcorrection would need increased pressure on the plunger. However, the amount of internal scar formation may differ from patient to patient.
Since connective tissue occupies the space between the microspheres and eventually makes up 80% of the implant, a few subsequent treatments (“Progressive enhancement”) are recommended rather than one “bulk” treatment. For example, a first implantation of 0.4 cc of ArteFill will often be sufficient for forehead lines or glabellar frown lines, one nasolabial fold, one upper or lower lip, both corners of the mouth, both marionette lines, or two neck folds. A second treatment may become necessary after 1–3 months in about 50% of the patients. In some patients desiring comprehensive treatment of acne scars, more than 30 cc of ArteFill were used over time.
Appropriate deep dermal placement with small aliquots of ArteFill and reevaluation of the results with a second treatment session as needed would optimize results utilizing this unique product in the clinical setting.
Conclusion
ArteFill’s unique mechanism of action and the appropriate injection techniques have been developed over the past two decades with its predecessor product Artecoll. Certain side effects and late complications of Artecoll [6] forced the manufacturer in the U.S. to make radical changes in ArteFill’s new formulation to achieve FDA approval as the first and only permanent dermal filler in 2006.
ArteFill is approved for the correction of nasolabial folds and has been used in over 15,000 patients since its U.S. market introduction in February 2007. No serious side effects have been reported to date according to the FDA’s MAUDE reporting database (www.fda.gov/cdrh).
References
Knapp TR, Kaplan EN, Daniels JR (1977) Injectable collagen for soft tissue augmentation. Plast Reconstr Surg 60:398–405
Knapp TR, Luck E, Daniels JR (1977) Behavior of solubilized collagen as a bioimplant. J Surg Res 23:96–105
Lemperle G, De Fazio S, Nicolau P (2006) ArteFill—a third generation dermal filler and tissue stimulator. Clin Plast Surg 33(4):551–565
Lemperle G, Duffy DM (2006) Treatment options of dermal filler complications. Aesthet Surg J 26:356–364
Lemperle G, Rullan PP, Gauthier-Hazan N (2006) Avoiding and treating dermal filler complications. Plast Reconstr Surg 118(3 Suppl):92S–107S
Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM (2009) Foreign body granulomas after all injectable dermal fillers. Part 1: possible causes. Plast Reconstr Surg 123:1842–1863
Lemperle G, Morhenn VB, Pestonjamasp V, Gallo R (2004) Migration studies and histology of injectable microspheres of different size in mice. Plast Reconstr Surg 113:1380–1390
Honma T, Hamasaki T (1996) Ultrastructure of multinucleated giant cell apoptosis in foreign body granuloma. Virchows Arch 428:165–1765
Lemperle G, Morhenn VB, Charrier U (2003) Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg 27:354–366
Nicolau PJ (2007) Long lasting and permanent fillers: biomaterial influence over host tissue response. Plast Reconstr Surg 119:2271–2286
Smith KC, Melnychuk M (2005) Five percent lidocain cream applied simultaneously to the skin and mucosa of the lips creates excellent anesthesia for filler injections. Dermatol Surg 31:1635–1637
Murray CA, Zloty D, Warshawski L (2005) The evolution of soft tissue fillers in clinical practice. Dermatol Clin 23:343–363
Cohen SR, Holmes RE (2004) Artecoll: a long-lasting injectable wrinkle filler material: report of a controlled, randomized, multicenter clinical trial of 251 subjects. Plast Reconstr Surg 114:964–967
Cohen SR, Berner CF, Busso M et al (2006) ArteFill®: a long-lasting injectable wrinkle filler material—summary of the U.S. Food and Drug Administration trials and a progress report on 4- to 5-year outcomes. Plast Reconstr Surg 118(1):64S–76S
Coleman SR (2002) Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J 22:555–557
Castro AC, Collares MV, Portinho CP, Dias PC, Pinto RA (2007) Extensive facial necrosis after infiltration of polymethyl-methacrylate. Braz J Otorhinolaryngol 73:850
Rullan PP (2004) Soft tissue augmentation using Artecoll: a personal experience. Facial Plast Surg 20:111–116
Lemperle G, Holmes RH, Cohen SR, Lemperle SM (2001) A classification of facial wrinkles. Plast Reconstr Surg 108:1735–1769
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: Drs. G. Lemperle and S. Lemperle are the developers of ArteFill®. Dr. N. Sadick is a clinical investigator of ArteFill. None of the authors own shares in Suneva Medical or have any kind of financial interest in ArteFill.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lemperle, G., Knapp, T.R., Sadick, N.S. et al. ArteFill® Permanent Injectable for Soft Tissue Augmentation: I. Mechanism of Action and Injection Techniques. Aesth Plast Surg 34, 264–272 (2010). https://doi.org/10.1007/s00266-009-9413-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-009-9413-1