Abstract
Accelerometers capture rapid changes in animal motion, and the analysis of large quantities of such data using machine learning algorithms enables the inference of broad animal behaviour categories such as foraging, flying, and resting over long periods of time. We deployed GPS-GSM/GPRS trackers with tri-axial acceleration sensors on common woodpigeons (Columba palumbus) from Hesse, Germany (forest and urban birds) and from Lisbon, Portugal (urban park). We used three machine learning algorithms, Random Forest, Support Vector Machine, and Extreme Gradient Boosting, to classify the main behaviours of the birds, namely foraging, flying, and resting and calculated time budgets over the breeding and winter season. Woodpigeon time budgets varied between seasons, with more foraging time during the breeding season than in winter. Also, woodpigeons from different sites showed differences in the time invested in foraging. The proportion of time woodpigeons spent foraging was lowest in the forest habitat from Hesse, higher in the urban habitat of Hesse, and highest in the urban park in Lisbon. The time budgets we recorded contrast to previous findings in woodpigeons and reaffirm the importance of considering different populations to fully understand the behaviour and adaptation of a particular species to a particular environment. Furthermore, the differences in the time budgets of Woodpigeons from this study and previous ones might be related to environmental change and merit further attention and the future investigation of energy budgets.
Significance statement
In this study we took advantage of accelerometer technology and machine learning methods to investigate year-round behavioural time budgets of wild common woodpigeons (Columba palumbus). Our analysis focuses on identifying coarse-scale behaviours (foraging, flying, resting) using various machine learning algorithms. Woodpigeon time budgets varied between seasons and among sites. Particularly interesting is the result showing that urban woodpigeons spend more time foraging than forest conspecifics. Our study opens an opportunity to further investigate and understand how a successful bird species such as the woodpigeon copes with increasing environmental change and urbanisation. The increase in the proportion of time devoted to foraging might be one of the behavioural mechanisms involved but opens questions about the costs associated to such increase in terms of other important behaviours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the behaviour of wild organisms in their natural environment, without biases introduced by the presence of observers, is essential to the advancement of ecological research (Cooke et al. 2004; Brown et al. 2013). Such knowledge can offer a unique opportunity to explore fundamental questions in ecology. For instance, it could allow investigating how an individual’s current behaviour affects future performance over the annual cycle (carry-over effects), the degree of flexibility or consistency in individual behaviour over different life-history stages (behavioural flexibility), or the effects of external conditions on behaviour among many other ecological and evolutionary questions (López-López 2016; Katzner and Arlettaz 2020; Williams et al. 2020). In the last 2 decades, we witnessed an unprecedented increase in studies using remote monitoring of animal behaviour, favoured by the development of innovative biologging technologies (Whitford and Klimley 2019; Katzner and Arlettaz 2020; Williams et al. 2020). The extraordinary amount of data gathered in this way allows novel insights in the behaviour of the tracked animals, features of the environments they move in, and provide information that can be used to identify biodiversity hotspots of conservation concern (Baylis et al. 2019; Williams et al. 2020).
Among biologging technologies, the use of accelerometers facilitates the investigation of animal behaviour, without the limitations imposed by animal visibility, rough terrain, remote locations, weather conditions, the geographical scales of space use, and the disturbance introduced by the presence of an observer (Shepard et al. 2008; Brown et al. 2013). The shape of accelerometer waveforms can be used to deduce specific animal behaviours, such as walking, resting, diving or flying, which are reflected in unique combinations of the accelerometer axes over time (Nathan et al. 2012; Shamoun-Baranes et al. 2012; Yeap et al. 2022). However, three-dimensional accelerometers can generate millions of data points in short periods of time. To analyse such large data sets, researchers started to employ machine learning methods. Machine learning algorithms are ideally suited to the task of extracting information from complex data and have been successfully used to distinguish among multiple classes of behaviours in wild mammals, birds, fish, and reptiles (e.g., Nathan et al. 2012; Valletta et al. 2017; Jeantet et al. 2018; Clarke et al. 2021; Clermont et al. 2021; Yu et al. 2021). However, studies conducted on birds concentrated on large species, mostly seabirds, with smaller species still underrepresented (Valletta et al. 2017; Wang 2019; but see Brown et al. 2022; Eisenring et al. 2022).
The way in which animals allocate resources among various conflicting needs is a central topic in ecology. Time is a limited resource leading to trade-offs, and its allocation to different behaviours (activity or time budget) may impact survival and reproduction (Pianka 1994; Christiansen et al. 2013; Quillfeldt et al. 2020). For instance, an increase in the time an animal invests in foraging will increase its access to energy. However, and consequently, it will reduce the time available for other activities such as breeding. Moreover, time budgets will reflect how the organism copes with factors like climate, seasonal changes in food availability, resource density, environmental heterogeneity, competition, predation risks, among others (Pianka 1994; Ropert-Coudert et al. 2004; Zhou et al. 2007). Time budgets will also manifest the necessities of phases like breeding, migration or wintering (Zhou et al. 2007; Brown et al. 2013; Bäckman et al. 2017a, b), and disclose the influence of factors like age, sex, or even anthropogenic disturbances (Fuchs and Caudill 2019; Colwill and Suchak 2021). In such a way, varying time budgets are a potent way of coping with a changing environment while retaining some degree of adaptation to it (Pianka 1994). Furthermore, as the time budget will be influenced by circadian and seasonal rhythms, as well as those from predators and potential prey, the time allocated to different activities may vary throughout the annual cycle (Pianka 1994; Bäckman et al. 2017a, b; Quillfeldt et al. 2020). A number of accelerometry studies examined time budgets in wild mammals, birds, fish, and reptiles (Brown et al. 2013; Shuert et al. 2019; Zhang et al. 2019; Cade et al. 2020; Fluhr et al. 2021), including some that used machine learning to classify behaviours (Valletta et al. 2017, e.g., Clermont et al. 2021; Lameris et al. 2021). Time budget studies conducted on birds also concentrated on large species, mostly seabirds, with smaller species (i.e. less than 1000 g) restricted to red-backed shrike (Lanius collurio) (Bäckman et al. 2017a, b), European nightjars (Caprimulgus europaeus) (Eisenring et al. 2022), dovekies (or little auks) (Alle alle) (Ste-Marie et al. 2022), streaked shearwaters (Calonectris leucomelas) (Garrod et al. 2021), and lesser black-backed gulls (Larus fuscus) (Brown et al. 2022) (species mentioned from lighter to heavier). Among them, only Brown et al. (2022) and Eisenring et al. (2022) used machine learning methods for behaviour classification, and only Bäckman et al. (2017a, b) investigated the activity patterns and their variability during the annual cycle in two red-backed shrike individuals monitored for over a year.
The common woodpigeon Columba palumbus (hereafter woodpigeon) is a medium-sized abundant Paleartic native bird (Columbiformes). Over the last decades, its population size and range have increased, and woodpigeons have increasingly moved into urban environments (Tomiałojć 1976; Bea et al. 2011; Schuster 2017). The species is resident in Southern Europe, while Western and Central European populations are short-distance partial migrants, and Eastern and Fennoscandian populations are strictly migratory (Butkauskas et al. 2019; Schumm et al. 2022). However, Western and Central European woodpigeons show a high degree of migration behaviour plasticity. In a previous study (Schumm et al. 2022), we showed that individuals may switch migratory strategies (resident vs. migrant) between years (i.e. facultative partial migrants). Woodpigeons forage in a number of vegetation types (Kułakowska et al. 2014), where they consume seeds, green plant material, fruits, and invertebrates (Gutiérrez-Galán et al. 2017; Dunn et al. 2018; Negrier et al. 2021). High-ingestion rates have been reported in woodpigeons, allowing individuals to fulfil their daily food needs in 2 h (Murton et al. 1963), and to spend, in particular habitats, up to 68% of their time resting (Murton and Isaacson 1962; Murton et al. 1964; Kenward and Sibly 1977).
In this study, we take advantage of accelerometers and machine learning methods to investigate year-round behavioural time budgets of wild woodpigeons. Our analysis focuses on identifying coarse-scale behaviours (foraging, flying, resting) using various machine learning algorithms. We aim to find patterns in behaviour that may differ between the breeding and wintering sites, both for short-distance migrant (all Central European) and resident individuals (Central and Southern European). Moreover, we seek to investigate differences in activity budgets between the sexes and among individuals. Our goal is to create a detailed basis for research into the behavioural adaptions, which may contribute to make woodpigeons a successful bird species (Brlík et al. 2021).
Materials and methods
Study sites, fieldwork procedures, tracker deployment, and data recording
We captured woodpigeons at three sites: the natural sciences campus of the University of Giessen and surrounding areas in Giessen (50° 35′ N, 8° 40′ E; hereafter Giessen; 12 females, 5 males), the Marburg Open Forest (50° 50′ N, 8° 39′ E; 1 female) both in Hesse, Germany, and the park Parque Florestal de Monsanto in Lisbon, Portugal (38° 43′ N, 9° 10′ W; hereafter Lisbon; 4 females, 3 males). We captured most birds with mist nets (mesh size 30 × 30 mm) with the exception of three birds captured in the vicinity of their nest with the help of a handheld net. We kept the time from capture to release mostly below 15 min and always below 20 min. We took extreme care to minimize stress to the captured birds, covering the head during handling. We determined the age of each bird by plumage examination. From the birds in Giessen and in the Marburg Open Forest, we collected blood samples (≤ 200 μl) from the brachial vein. We detected no adverse effects related to blood sampling. From the birds in Lisbon, we sampled a few body feathers. We used blood and feather samples for molecular sexing with standard methods (Griffiths et al. 1998).
We deployed OrniTrack-15 solar-powered GPS (Global Positioning System)-GSM (Global System for Mobile Communication)/GPRS (General Packet Radio Service) trackers (Ornitela, Lithuania) on the back of the birds using a 4-mm wide Teflon ribbon harness (Fig. S1, Supplementary Information). The trackers used (17 g, 58 × 25 × 14 mm) represented between 2.7 and 4.5% of the woodpigeon’s body mass (range for the deployed birds: 380 to 635 g) in agreement with the maximum recommended tracker masses for pigeons (Tian et al. 2020). From June 2019 to July 2021, the trackers recorded time of day and detailed location (longitude, latitude) at sampling intervals dependent on battery charge: 5 min, when the battery was > 75% full, 30 min, for a battery charge of 50–75%, 4 h, for a battery charge of 25–50%, or 8 h, when the charge was < 25%. We set the trackers to sleep from civil dusk to civil dawn when the centre of the Sun’s disc goes 6° below the horizon. This allowed us to maximize battery charge but no data were recorded during the night. In this way, we recorded 23,821 GPS locations corresponding to the bird from the Marburg Open Forest, 477,589 locations corresponding to the birds from Giessen, and 138,263 corresponding to those from Lisbon.
The trackers also recorded tri-axial acceleration (three axes perpendicular to each other: x, y, z, i.e. surge, sway, heave; units, g/1000) during 10 s after each GPS fix, at a sampling rate of 20 Hz. This resulted in 201 acceleration measurements taken for each axis in each measurement interval i.e. one measurement during the GPS fix and 200 after that. Hereafter, we refer to a 10-s measurement interval as a burst.
It was not possible to record data blind because our study involved focal animals in the field.
Ground-truthed acceleration data
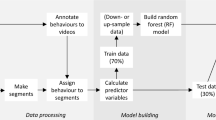
We inferred classes of behaviour from acceleration data using an adaptation of the method described in detail by Rast et al. (2020). To train algorithms for pattern recognition and data classification, we selected bursts of acceleration data for which the context of the bird was in (e.g. speed, location) allowed delimiting likely behaviours e.g. flying. We opted for this method, as direct observation of the tracked individuals was not a feasible option. Woodpigeons, in the forest and environments with large trees we investigated, are elusive and cryptic. In open fields, they were easily disturbed and interrupted their natural behaviour, even when we attempted observations at a distance of 150 m or more. Thus, following the methodology from previous studies (Yoda et al. 2001; Tsuda et al. 2006; Zimmer et al. 2011; Williams et al. 2014), we used GPS locations and speed data to identify the burst containing potential training data for particular behaviours. We used QGIS 3.4 (QGIS Development Team) and detailed base maps (topographic charts 1:25000, Hessische Verwaltung für Bodenmanagement und Geoinformation, http://www.gds-srv.hessen.de/cgi-bin/lika-services/de-viewer/access/ogc-free-maps.ows) to plot GPS locations e.g. Fig. S2 (Supplementary Information). We selected a burst as a candidate for: (1) flying behaviour, when the speed measured during the GPS fix was 10 m s−1 to 24 m s−1, values previously recorded in free-flying homing pigeons Columba livia (Sankey et al. 2019), (2) foraging behaviour, when the GPS fixes were located on parcels with cereal stubbles, previously found to be woodpigeon preferred foraging-sites (Murton et al. 1964), and (3) resting behaviour, when fixes were located on bare ploughed land, where woodpigeon rest and do not search for food (Murton et al. 1964). Additionally, we visited the locations corresponding to the training data candidates to check for their potential suitability for the corresponding behaviour and to distinguish between cereal stubbles and bare ploughed land. After selecting a candidate burst, we plotted the acceleration data (x, y, z) against time and compared the graph with similar ones previously published (Hedrick et al. 2004; Ropert-Coudert et al. 2004; Gómez Laich et al. 2008; Shepard et al. 2008; Wilson et al. 2008; Halsey et al. 2009; Holland et al. 2009; Sakamoto et al. 2009; Nathan et al. 2012; Shamoun-Baranes et al. 2012; Sommerfeld et al. 2013; Bom et al. 2014; Resheff et al. 2014; Collins et al. 2015; Garrod et al. 2021; Yeap et al. 2022). Finally, we selected bursts (51 for foraging, 55 for flying, and 49 for resting) as the input for the model training, whose plots showed a clear signal of a particular behaviour. We provide examples of the selected bursts in Fig. 1. All these behaviour classifications may include other related behaviours that could not be separated from the main ones e.g. some degree of walking during foraging, take-off, and landing during flights.
Representative bursts corresponding to dynamic acceleration during foraging (A), flying (B), and resting (C) by common woodpigeon (Columba palumbus). We represented acceleration (in g/1000, as received from the sensor) using coloured continues lines: blue in for the surge (X) axis, black for sway (Y), and grey for heave (Z). The graph x-axis corresponds to the 201 measurements recorded during the burst i.e. 1 measurement during the GPS fix and 200 after that
Data preparation and algorithms used
Using the sum_data function in the R package accelerateR (Rast et al. 2020), we calculated summary statistics (mean, standard deviation, inverse coefficient of variation, variance, skewness, and kurtosis) from the raw acceleration data, separately for each burst and axis, to use as predictors for the algorithms. We performed all data transformations in R (R Core Team 2021) and RStudio (RStudio Team 2021).
We used three machine learning algorithms to classify the acceleration data: Random Forest, Support Vector Machines, and Extreme Gradient Boosting. Random Forest selects a random subset of predictors to fit a tree, a procedure that is repeated a number of times, and the final prediction is the result of all trees combined by a majority rule (Breiman 2001). We implemented Random Forest in the R package randomForest (Liaw and Wiener 2002) using the default number of trees (500). Support Vector Machines is a method for two-group classification problems in which input vectors are non-linearly mapped to a hyperplane. Classification of new groups is subsequently based on their relative position to the hyperplane. To account for multiple classes, Support Vector Machines constructs additional hyperplanes between the classes (Cortes and Vapnik 1995; Wang 2019). We implemented Support Vector Machines in the R package e1071 (Meyer et al. 2017) setting the kernel type to “radial”. Extreme Gradient Boosting (Chen and Guestrin 2016) is a scalable implementation of the gradient boosting concept (Friedman 2001) used for regression and classification problems. Extreme Gradient Boosting produces in a stage-wise fashion a prediction model, typically in the form of an ensemble of decision trees, which are generalized by allowing optimization of an arbitrary differentiable loss function (Chen and Guestrin 2016). We implemented Extreme Gradient Boosting in the R package xgboost (Chen et al. 2015) setting the number of iterations (nrounds) to 20.
Training data and model evaluation
Previous studies showed an algorithm-improved performance with larger sample sizes (Zhang et al. 1998; Rast et al. 2020). For this reason, and following the procedure detailed described in Rast et al. (2020), we applied a moving window to every acceleration burst to increase the sample size of our training data. We used a window that reduced the amount of data of a burst from the original 201 acceleration measurements down to a subset of 40 (i.e. covering 2 s). Then, we calculated the summary statistics for the subset. As the next step, we slid the window one position and calculated the summary statistics for the new subset. We repeated this procedure, sliding the window until it included the last measurement of the burst. Consequently, we were able to calculate a larger number of predictor-set representatives of the same burst, and, at the same time, preserve the order of the acceleration measurements for a specific behaviour (Rast et al. 2020).
We first trained the models using a randomly selected 70% of the ground-truthed data (training data set). Then, we inferred the behaviour of the remaining 30% of the ground-truthed data (validation data set), assigning a specific behaviour (foraging, flying, resting). We accepted only behaviour assignments with a probability ≥ 0.7, classifying all assignments below this threshold as “non-annotated”. By introducing the category “non-annotated”, we account for a limitation in our training data that may not include the full range of behaviours present in woodpigeons e.g. fighting or walking. Individuals displaying behaviours not included in our model could cause an incorrect classification. However, we expect that in such cases, classifications would be assigned a low probability and consequently avoid these errors by implementing the threshold. Likewise, this method allows that when an individual changed its behaviour during a burst, the behaviour assignments also had a probability below the threshold and thus, was classified as “non-annotated”. The three algorithms showed a high consistency among them (Table S1 in the Supplementary Information).
Application to all individuals
After training all three classification models, we applied the trained models to classify the acceleration data of every deployed bird (Table S2 in the Supplementary Information). In this case, the acceleration data of each individual was analysed using a non-overlapping window of 40 measurements of acceleration data (x, y, z) starting on a GPS fix. This resulted in five acceleration measurements subsets per burst, each of which resulted in a behaviour classification output (foraging, flying, resting, or non-annotated) per model. Next, we generated a consensus behaviour summarising the outputs of the three models. If all models agreed on one particular behaviour, then that was considered the consensus behaviour, which was used in further statistical analyses. Otherwise, the consensus behaviour for that particular window was set as “non-annotated”, and used in that way in further statistical analyses.
Data summarising and generalized additive models
After classification, we summarised the consensus behaviours by month and by stages of the annual cycle. For this, we used contingency tables in the R programming language to calculate the proportion of time spent in each behaviour (foraging, flying, resting, or non-annotated) during each month or stage. We considered two stages: (1) breeding, including the behaviours carried out during the breeding season, i.e. April to August (Bezzel 1985) and (2) winter, for those behaviours carried out from November to February. When summarising by stages, in order to be conservative, we excluded the data from transitional months, as some individuals can start breeding earlier than April, while others continue to breed until quite late in the season i.e. after August. Due to incomplete records related to battery charge, we excluded some data from the summaries. We excluded the data of a particular individual during a specific month if (1) the number of occurrences of one of the behaviours was less than five (18 cases) or (2) the sum of all behaviours was < 500 (20 cases). In those cases, we considered the data not representative of the month investigated.
To account for the non-linear relationship between the proportion of each behaviour classification (foraging, flying, resting, or non-annotated) and time (Fig. 2), we ran generalized additive models in the R package mgcv (Wood et al. 2016). We modelled the proportion of time spent in each behaviour as a smooth function of the independent variable time, including the site where the birds were captured (Marburg Open Forest, Giessen, Lisbon; hereafter, site), their age (adult, juvenile; juvenile: all first-year individuum), and sex, and whether they were migrant or resident birds (hereafter, migratory status or migrant/resident, depending on the context) as factors (gam(foraging/flying/resting_prop ~ s(month) + site + age + sex + migratory_status). We checked all generalized additive models for model convergence and random distribution of residuals. We reported statistics (effective degrees of freedom, edf, and p values) for the generalized additive models run separately for each parameter. In the dredge function in the R package MuMIn (Barton 2022), all possible candidate models were tested using each unique linear combination of factors. The best models are then selected based on delta Akaike information criteria scores less than or equal to two. We classified individual woodpigeons in this study as migrant or resident following the results of our previous study investigating individual-specific migration decision and phenology (Schumm et al. 2022). See Table S2 in the Supplementary Information for individual classification. Please note that sample sizes differ between Schumm et al. (2022) and this study, as our previous work also included ring recovery data and tracking data from woodpigeons for which no acceleration data was available.
Generalized additive model fits for the proportion of time (%) spent by common woodpigeon (Columba palumbus) in each behaviour along the year. Lines correspond to the estimated smoothing curves. Grey shadings show the generalized additive model 95% confidence interval for the mean shape of the effect. n-a, non-annotated
To compare the proportion of time spent in each behaviour during the breeding season and winter (hereafter, season), we performed 3-way ANOVAs in the aov function in the base R package (R Development Core Team 2022). We included site, sex, and migratory status as factors. In this case, age was not included, as only data from one juvenile was available for winter, due to low battery charge in the second juvenile individual. We first included interaction effects in the models but removed them if they were not significant.
Due to incomplete records for some of the individuals, related to low battery charge or death, the sample sizes differ for the different tests, month, sites, ages, sexes, migratory status, or their combination. For this, we provide detailed samples sizes in Tables S2 to S9 in the Supplementary Information.
Results
Differences between the breeding season and winter
Woodpigeons spent more time foraging during the breeding season than in winter (Figs. 3A and 4). Also, birds from different sites showed differences in the time invested in foraging (Table 1). During the breeding season, the time spent foraging was lowest in the bird from the Marburg Open Forest (14%), higher in those birds from Giessen (20 to 22%), and highest in those from Lisbon (28 to 29%; Fig. 4A, Table S3 in the Supplementary Information). In winter, the bird from the Marburg Open Forest also invested the least time foraging (7%), followed again by birds from Giessen (9% to 14%) and Lisbon (8 to 16%; Fig. 4B, Table S4).
Violin and boxplots describing the proportion of time spent by migrant and resident common woodpigeons (Columba palumbus) in each behaviour during the breeding season and winter. The proportions of time foraging (A), flying (B), and resting (C) are expressed in percentages. Violin plots delineate the kernel probability densities, while boxplots illustrate medians and inter-quartile ranges (IQR) with whiskers denoting 1.5 × IQR
We did not find any differences in the proportion of time spent flying during the breeding season compared to winter (Figs. 3B and 4, Table 1). However, woodpigeons from Lisbon and the Marburg Open Forest spent less time flying (1 to 2.3%, 1.6 to 1.7%, respectively) than those from Giessen (2.1 to 3.3%; Fig. 4, Tables S3, S4). Particularly, males from Giessen spent significantly more time (3.3%) than females (2.4%) flying during the breeding season (Table 1, S3, Fig. 4A).
Woodpigeons spent more time resting during winter (67 to 81%) than during the breeding season (54% to 70%; Figs. 3C and 4, Table 1).
Year-round behavioural time budget
In January (winter), woodpigeons spent little time foraging (7%), resting for most of the time (80%). In May, during the breeding season, the opposite was true, when woodpigeons devoted the highest amount of time to foraging (26%) and the lowest to resting (56%). Woodpigeons flew 1–5% of the time (Fig. 2).
Generalized additive models revealed significant effects of site and age on the proportion of time foraging over the year (Table 2). Site was the strongest predictor, with the highest proportion of foraging observed in woodpigeons from Lisbon, followed by those from Giessen (Fig. 5, Table S7). During most months, adults spent more time foraging than juveniles, however this result, as well as following ones involving juveniles, should be taken with caution as the juvenile sample sizes were low (Table S8). Model selection retained four models, in which month, site, and age always showed an effect on the proportion of time foraging, while sex and migratory status had an effect only in two of the models (Fig. S3). Males spent more time than females foraging in 7 months, with the highest differences observed in May and July (Fig. S4, Table S6). Resident woodpigeons spent more time foraging except during October and November, when migrants invested slightly more time in this behaviour (Fig. S5, Table S9).
Generalized additive models also revealed significant effects of site, age, and sex on the proportion of time flying over the year (Table 2). Site was the strongest predictor, with the highest proportion of time flying observed in woodpigeons from Giessen, followed by those from Lisbon, and with the lowest proportion observed in the Marburg Open Forest (Table S7). For the proportion of time flying, model selection retained two models, one of which included month, site, migratory status, and age, while the other model retained all the factors but migratory status (Fig. S3). In most months, males and juveniles spent the highest proportion of time flying (Tables S6, S8). During March to May, and October to November, migratory birds spent more time flying than residents (Table S9).
With respect to the proportion of time resting, generalized additive models indicated significant effects of site, age, and sex (Table 2). Site was the strongest predictor, with the highest proportion of resting observed in the woodpigeon from the Marburg Open Forest, followed by those from Giessen and Lisbon (Table S7). During most months, juveniles spent more time resting than adults (Table S8). Model selection retained only one model for the proportion of resting, including all factors except the migration status (Fig. S3).
Discussion
We took advantage of biologging technologies, particularly tri-axial acceleration, and machine learning to classify and quantify coarse-scale behaviours of wild woodpigeons year-round. This approach allowed us to examine how woodpigeons allocated time to foraging, flying, and resting over the year.
Woodpigeons in our study spent more time foraging during the breeding season (14 to 29 % of daytime hours) than in winter (7 to 16 %; Figs. 3A and 4). The higher proportion of time allocated to foraging during the breeding season (Fig. 5) is an expected pattern. In general, breeding birds face an increase in energy demands particularly when adults provision the young (e.g. Ettinger and King 1980; Green et al. 2009). Thus, the peak observed in Fig. 5 reflects an increase in food search related to breeding effort. Even more, our results show two peaks (Fig. 5), which coincide with the peaks of breeding activity previously reported for woodpigeons (early and late or first and second broods; Cramp 1958, 1972; Murton 1958; Wittenberg 1980; Herkenrath 1989; Tomiałojć 1999; Slater 2001). Our results also show the peaks when considering the sexes separately (Fig. S8), with males having higher peaks of foraging activity than females during the breeding season. The higher peaks in males (in May and July) could be explained by the incubation pattern of this species. Males incubate for about 7 h, while females incubate around 9 h per day. Consequently, males have 2 h more for foraging daily (Murton and Isaacson 1962; Bezzel 1985).
Our results contrast to previous findings in Carlton, an area devoted to arable farming in Suffolk, UK (Murton and Isaacson 1962; Murton et al. 1963, 1964, 1971). In Carlton, woodpigeons allocated more time to foraging than in our study sites, with the highest proportion of foraging reported for January (94 to 98 %) and the lowest for May (32 to 57 %; Murton et al. 1964). The difference between our results and those from Murton et al. (1963, 1964) could originate in the different methods and sampling rates used. Murton and colleagues conducted fortnightly daytime standard walks and counts (Murton et al. 1964), while we recorded data of tracked birds every day, for 2 years, at sampling rates ranging from 5 min (mostly) to 8 h, dependent on battery charge. The higher sampling rate in our study may have revealed patterns not captured by the fortnightly walks in Carlton. The difference could also be due to an assumption made by Murton et al. (1963); if a flock of 100 woodpigeons was observed where 98 % were foraging and 2 % were resting, then each individual on average was supposed to spent 2 % of its time on the foraging ground in activities other than foraging. However, as Kenward and Sibly (1977) found, activities carried out by woodpigeons vary along the day, and thus, the assumption made by Murton et al. (1963) could be flawed. A third possible explanation is that the discrepancies are actually related to the different study sites. Already, Murton et al. (1963) and Kenward and Sibly (1977) mentioned that the proportion of resting behaviour, and consequently to other behaviours, varies among sites. In our study, we found that woodpigeons from different sites showed significant variation in the time allocated to foraging during the breeding and winter seasons (Table 1). Also, the generalized additive models in our study revealed that site was the strongest predictor of the proportion of time that woodpigeons spent foraging over the year (Table 2). Thus, variation in time budgets might be expected among sites. The reason for this may possibly be a variation among sites in food availability, accessibility, or both, and thus the energy the birds can obtain during foraging. This variation merits further investigation and will be part of our next research on woodpigeons.
The proportion of time woodpigeons spent foraging was lowest in the forest habitat from the Marburg Open Forest, higher in the urban habitats of Giessen, where birds foraged on nearby arable fields during the day (Schumm et al. 2022), and highest in the urban park in Lisbon (see Fig. S6 for an overview of the habitats used by the woodpigeons of the different sites). We observed this pattern both during the breeding season and in winter (Fig. 4). This result could reflect both variation in food availability and accessibility among sites and the cost of adaptation to urban environments (synurbanisation) in woodpigeons. Originally, a forest species, woodpigeons have adapted to urban and suburban habitats in Western and Central Europe since the early nineteenth century, and more recently, in Eastern Europe (Tomiałojć 1976; Witt et al. 2005; Bea et al. 2011; Fey et al. 2015; Schuster 2017). Increasing urbanisation is advantageous for some bird species, shows no effect for others, or can be detrimental (reviewed in Chace and Walsh 2006; Chamberlain et al. 2009; Seress and Liker 2015; Isaksson 2018). As a result, activity budgets can be affected in birds foraging in urban areas (Chace and Walsh 2006). A number of factors may play a role in the response of a particular species to urban food resources: (1) the presence and patch size of remnant native vegetation, (2) competition with other species with longer cohabitation histories with humans, (3) the presence and density of non-native predators, (4) the structure and floristic characteristics of planted vegetation, and (5) the amount of supplementary feeding by humans, among others (reviewed in Chace and Walsh 2006). Differences in small-scale habitat and climate, variation in food availability, and accessibility among sites, the actual diet of the tracked birds, or the physiological consequences of the types of food available may also influence time budgets. However, we did not investigate these factors in our study, rendering our interpretation as only a possible one. Also, we tracked only one forest individual, but this bird showed interesting differences to the birds breeding in Giessen (Fig. 5). Therefore, future studies should include more forest woodpigeons to confirm these differences. Future work on the energy budgets of tracked woodpigeons and their habitat use may shed more light on the causes of the altered activity budgets observed in our urban individuals.
Conclusions and outlook
Tri-axial acceleration and machine learning allowed us to investigate year-round behavioural time budgets of wild woodpigeons. The long-term and detailed information gathered, together with the knowledge gained about time allocation to the different behaviours, constitute the basis for future research into the behavioural adaptions of woodpigeons to the environment. The time budgets we recorded contrast to previous findings in woodpigeons (Murton and Isaacson 1962; Murton et al. 1963, 1964, 1971) and reaffirm the importance of considering different populations to fully understand the behaviour and adaptation of a particular species to a particular environment. Furthermore, the differences in the time budgets of woodpigeons from this study and those from the 1950s and 1960s might be related to environmental change and merit further attention and the future investigation of energy budgets. The latter will also allow to fully understand the cost of adaptation to urban environments, suggested by the differences we recorded among urban and forest woodpigeons. Furthermore, our results open an opportunity to further investigate how a successful bird species such as the woodpigeon copes with increasing environmental change and urbanisation. The increase in the proportion of time devoted to foraging might be one of the behavioural mechanisms involved but opens questions about the costs associated to such increase in terms of other important behaviours.
Our present study was restricted to the classification and quantification of coarse-scale behaviours of wild woodpigeons. However, Murton et al. (1964) showed that for the woodpigeons from Carlton drinking accounted for up to 9% of the proportion of time in summer. Unfortunately, we only identified four confirmed bursts for drinking behaviour (Fig. S7), which were not enough to train the algorithms. Territorial calls, bowing and aerial displays, actual intra- and inter-specific (with Stock Doves Columba oenas) fighting following aggressive posturing to intruders, courtship feeding, copulation, caressing, nest building and maintenance, incubation, brooding, and care of the young are also important behaviours during the breeding season (Murton and Isaacson 1962). The future use of animal-borne video cameras at the same time of tri-axial acceleration recordings, recently used in larger birds (Del Caño et al. 2021), may provide an opportunity to peer in greater detail into the activity budgets of medium-sized birds as the woodpigeons. However, further miniaturization of the necessary instruments (32 g in Del Caño et al. 2021) is still required for them to be suitable for deployment on woodpigeons.
Data availability
The data sets supporting the conclusions of this study are archived in the Movebank repository (IDs, 746410443, 897868497; https://www.movebank.org/). All other data supporting the conclusions of this article are included or cited within the article and its Additional file 1.
References
Bäckman J, Andersson A, Alerstam T, Pedersen L, Sjöberg S, Thorup K, Tøttrup AP (2017a) Activity and migratory flights of individual free-flying songbirds throughout the annual cycle: method and first case study. J Avian Biol 48:309–319
Bäckman J, Andersson A, Pedersen L, Sjöberg S, Tøttrup AP, Alerstam T (2017b) Actogram analysis of free-flying migratory birds: new perspectives based on acceleration logging. J Comp Physiol A 203:543–564
Barton K (2022) MuMIn: multi-model inference. R package version 1(46) https://cran.r-project.org/web/packages/MuMIn/index.html
Baylis AMM, Tierney M, Orben RA, Warwick-Evans V, Wakefield E, Grecian WJ, Trathan P, Reisinger R, Ratcliffe N, Croxall J, Campioni L, Catry P, Crofts S, Boersma PD, Galimberti F, Granadeiro J, Handley J, Hayes S, Hedd A, Masello JF, Montevecchi WA, Pütz K, Quillfeldt P, Rebstock GA, Sanvito S, Staniland IJ, Brickle P (2019) Important at-sea areas of colonial breeding marine predators on the Southern Patagonian Shelf. Scientific Reports 9:8517
Bea A, Svazas S, Grishanov G, Kozulin A, Stanevicius V, Astafieva T, Olano I, Raudonikis L, Butkauskas D, Sruoga A (2011) Woodland and urban populations of the woodpigeon Columba palumbus in the eastern Baltic region. Ardeola 58:315–321
Bezzel E (1985) Kompendium der Vögel Mitteleuropas. AULA-Verlag, Wiesbaden, Germany
Bom RA, Bouten W, Piersma T, Oosterbeek K, van Gils JA (2014) Optimizing acceleration-based ethograms: the use of variable-time versus fixed-time segmentation. Mov Ecol 2:art6
Breiman L (2001) Random forests. Mach Learn 45:5–32
Brlík V, Šilarová E, Škorpilová J et al (2021) Long-term and large-scale multispecies dataset tracking population changes of common European breeding birds. Sci Data 8:21
Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP (2013) Observing the unwatchable through acceleration logging of animal behavior. Anim Biotelem 1:art20
Brown JM, Bouten W, Camphuysen KCJ, Nolet BA, Shamoun-Baranes J (2022) Acceleration as a proxy for energy expenditure in a facultative-soaring bird: comparing dynamic body acceleration and time-energy budgets to heart rate. Funct Ecol 36:1627–1638
Butkauskas D, Švažas S, Bea A et al (2019) Designation of flyways and genetic structure of woodpigeon Columba palumbus in Europe and Morocco. Eur J Wildlife Res 65:91
Cade DE, Levenson JJ, Cooper R, de la Parra R, Webb DH, Dove ADM (2020) Whale sharks increase swimming effort while filter feeding, but appear to maintain high foraging efficiencies. J Exp Biol 223:jeb224402
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landscape Urban Plan 74:46–69
Chamberlain DE, Cannon AR, Mp T, Di L, Bj H, Kj G (2009) Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151:1–18
Chen T, Guestrin C (2016) XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. Association for Computing Machinery, San Francisco, CA, USA, pp 785–794
Chen T, He T, Benesty M, Khotilovich V, Tang Y, Cho H, Chen K (2015) Xgboost: extreme gradient boosting. R package version 1(4):1–4 https://cran.r-project.org/web/packages/xgboost/index.html
Christiansen F, Rasmussen MH, Lusseau D (2013) Inferring activity budgets in wild animals to estimate the consequences of disturbances. Behav Ecol 24:1415–1425
Clarke TM, Whitmarsh SK, Hounslow JL, Gleiss AC, Payne NL, Huveneers C (2021) Using tri-axial accelerometer loggers to identify spawning behaviours of large pelagic fish. Mov Ecol 9:26
Clermont J, Woodward-Gagné S, Berteaux D (2021) Digging into the behaviour of an active hunting predator: arctic fox prey caching events revealed by accelerometry. Mov Ecol 9:58
Collins PM, Green JA, Warwick-Evans V, Dodd S, Shaw PJA, Arnould JPY, Halsey LG (2015) Interpreting behaviors from accelerometry: a method combining simplicity and objectivity. Ecol Evol 5:4642–4654
Colwill RM, Suchak M (2021) Activity budget. In: Vonk J, Shackelford T (eds) Encyclopedia of animal cognition and behavior. Springer, Cham, pp 1–7
Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Kuchel LJ, Wolcott TG, Butler PJ (2004) Biotelemetry: a mechanistic approach to ecology. Trends Ecol Evol 19:335–343
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20:273–297
Cramp S (1958) Territorial and other behaviour of the woodpigeon. Bird Study 5:55–66
Cramp S (1972) The breeding of urban woodpigeons. Ibis 114:163–171
Del Caño M, Quintana F, Yoda K, Dell’Omo G, Blanco GS, Gómez-Laich A (2021) Fine-scale body and head movements allow to determine prey capture events in the Magellanic penguin (Spheniscus magellanicus). Mar Biol 168:84
Dunn JC, Stockdale JE, Moorhouse-Gann RJ, McCubbin A, Hipperson H, Morris AJ, Grice PV, Symondson WOC (2018) The decline of the turtle dove: dietary associations with body condition and competition with other columbids analysed using high-throughput sequencing. Mol Ecol 27:3386–3407
Eisenring E, Eens M, Pradervand J-N, Jacot A, Baert J, Ulenaers E, Lathouwers M, Evens R (2022) Quantifying song behavior in a free-living, light-weight, mobile bird using accelerometers. Ecol Evol 12:e8446
Ettinger AO, King JR (1980) Time and energy budgets of the willow flycatcher (Empidonax traillii) during the breeding season. Auk 97:533–546
Fey K, Vuorisalo T, Lehikoinen A, Selonen V (2015) Urbanisation of the wood pigeon (Columba palumbus) in Finland. Landscape Urban Plan 134:188–194
Fluhr J, Benhamou S, Peyrusque D, Duriez O (2021) Space use and time budget in two populations of griffon vultures in contrasting landscapes. J Raptor Res 55:425–437
Friedman JH (2001) Greedy function approximation: a gradient boosting machine. Ann Stat 29:1189–1232
Fuchs NT, Caudill CC (2019) Classifying and inferring behaviors using real-time acceleration biotelemetry in reproductive steelhead trout (Oncorhynchus mykiss). Ecol Evol 9:11329–11343
Garrod A, Yamamoto S, Sakamoto KQ, Sato K (2021) Video and acceleration records of streaked shearwaters allows detection of two foraging behaviours associated with large marine predators. PLoS ONE 16:e0254454
Gómez Laich A, Wilson RP, Quintana F, Shepard ELC (2008) Identification of imperial cormorant Phalacrocorax atriceps behaviour using accelerometers. Endanger Species Res 10:29–37
Green JA, Boyd IL, Woakes AJ, Warren NL, Butler PJ (2009) Evaluating the prudence of parents: daily energy expenditure throughout the annual cycle of a free-ranging bird, the macaroni penguin Eudyptes chrysolophus. J Avian Biol 40:529–538
Griffiths R, Double M, Orr K, Dawson R (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1076
Gutiérrez-Galán A, González CA, Maroto de Mercado J (2017) Woodpigeon Columba palumbus diet composition in Mediterranean Southern Spain. Ardeola 64:17–30
Halsey LG, Portugal SJ, Smith JA, Murn CP, Wilson RP (2009) Recording raptor behavior on the wing via accelerometry. J Field Ornithol 80:171–177
Hedrick TL, Usherwood JR, Biewener AA (2004) Wing inertia and whole-body acceleration: an analysis of instantaneous aerodynamic force production in cockatiels (Nymphicus hollandicus) flying across a range of speeds. J Exp Biol 207:1689–1702
Herkenrath P (1989) Die Brutsaison einer städtischen Population der Ringeltaube (Columba palumbus L.). Z Jagdwiss 35:119–124
Holland RA, Wikelski M, Kümmeth F, Bosque C (2009) The secret life of oilbirds: new insights into the movement ecology of a unique avian frugivore. PLoS ONE 4:e8264
Isaksson C (2018) Impact of urbanization on birds. In: Tietze DT (ed) Bird species. How they arise, modify and vanish. Springer Open, Cham, Switzerland, pp 235–257
Jeantet L, Dell'Amico F, Forin-Wiart M-A et al (2018) Combined use of two supervised learning algorithms to model sea turtle behaviours from tri-axial acceleration data. J Exp Biol 221:jeb177378
Katzner TE, Arlettaz R (2020) Evaluating contributions of recent tracking-based animal movement ecology to conservation management. Front Ecol Evol 7:519
Kenward R, Sibly R (1977) A woodpigeon (Columba palumbus) feeding preference explained by a digestive bottle-neck. J Appl Ecol 14:815–826
Kulakowska KA, Kulakowski TM, Inglis IR, Smith GC, Haynes PJ, Prosser P, Thorbek P, Sibly RM (2014) Using an individual-based model to select among alternative foraging strategies of woodpigeons: Data support a memory-based model with a flocking mechanism. Ecol Model 280:89–101
Lameris TK, Dokter AM, van der Jeugd HP, Bouten W, Koster J, Sand SHH, Westerduin C, Nolet BA (2021) Nocturnal foraging lifts time constraints in winter for migratory geese but hardly speeds up fueling. Behav Ecol 32:539–552
Liaw A, Wiener M (2002) Classification and regression by randomforest. R News 2:18–22
López-López P (2016) Individual-based tracking systems in ornithology: welcome to the era of big data. Ardeola 63:103–136
Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F (2017) e1071: Misc functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien. R package version 3(5):1 https://cran.r-project.org/web/packages/e1071/index.html
Murton R (1958) The breeding of woodpigeon populations. Bird Study 5:157–183
Murton R, Isaacson A (1962) The functional basis of some behaviour in the woodpigeon Columba palumbus. Ibis 104:503–521
Murton R, Isaacson A, Westwood N (1963) The feeding ecology of the woodpigeon. Brit Birds 56:345–375
Murton R, Isaacson A, Westwood N (1971) The significance of gregarious feeding behaviour and adrenal stress in a population of wood-pigeons Columba palumbus. J Zool 165:53–84
Murton RK, Westwood NJ, Isaacson AJ (1964) The feeding habits of the woodpigeon Columba palumbus, stock dove C. oenas and turtle dove Streptopelia turtur. Ibis 106:174–188
Nathan R, Spiegel O, Fortmann-Roe S, Harel R, Wikelski M, Getz WM (2012) Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: general concepts and tools illustrated for griffon vultures. J Exp Biol 215:986–996
Negrier C, Fantinati M, Jouglar J-Y, Lyazrhi F, Cohou V, Priymenko N (2021) Dietary regimen of the woodpigeon (Columba palumbus). J Anim Physiol Anim Nutr 105:376–384
Pianka ER (1994) Evolutionary ecology. Harper Collins, New York
Quillfeldt P, Weimerskirch H, Delord K, Cherel Y (2020) Niche switching and leapfrog foraging: movement ecology of sympatric petrels during the early breeding season. Mov Ecol 8:e23
R Development Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Rast W, Kimmig SE, Giese L, Berger A (2020) Machine learning goes wild: using data from captive individuals to infer wildlife behaviours. PLoS ONE 15:e0227317
Resheff YS, Rotics S, Harel R, Spiegel O, Nathan R (2014) AcceleRater: a web application for supervised learning of behavioral modes from acceleration measurements. Mov Ecol 2:27
Ropert-Coudert Y, Gremillet D, Kato A, Ryan PG, Naito Y, Le Maho Y (2004) A fine-scale time budget of Cape gannets provides insights into the foraging strategies of coastal seabirds. Anim Behav 67:985–992
RStudio Team (2021) R Studio: integrated development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Sakamoto KQ, Sato K, Ishizuka M, Watanuki Y, Takahashi A, Daunt F, Wanless S (2009) Can ethograms be automatically generated using body acceleration data from free-ranging birds? PLoS ONE 4:e5379
Sankey DWE, Shepard ELC, Biro D, Portugal SJ (2019) Speed consensus and the ‘Goldilocks principle’ in flocking birds (Columba livia). Anim Behav 157:105–119
Schumm YR, Masello JF, Cohou V, Mourguiart P, Metzger B, Rösner S, Quillfeldt P (2022) Should I stay or should I fly? Migration phenology, individual-based migration decision and seasonal changes in foraging behaviour of common woodpigeons. Sci Nat 109:44
Schuster S (2017) Verhaltensänderungen bei Ringeltauben Columba palumbus im Voralpenraum. Ornithol Jh Bad-Württ 33:71–80
Seress G, Liker A (2015) Habitat urbanization and its effects on birds. Acta Zool Hung 61:373–408
Shamoun-Baranes J, Bom R, van Loon EE, Ens BJ, Oosterbeek K, Bouten W (2012) From sensor data to animal behaviour: an oystercatcher example. PLoS ONE 7:e37997
Shepard ELC, Wilson RP, Quintana F et al (2008) Identification of animal movement patterns using tri-axial accelerometry. Endanger Species Res 10:47–60
Shuert CR, Pomeroy PP, Twiss SD (2019) Coping styles in capital breeders modulate behavioural trade-offs in time allocation: assessing fine-scale activity budgets in lactating grey seals (Halichoerus grypus) using accelerometry and heart rate variability. Behav Ecol Sociobiol 74:8
Slater P (2001) Breeding ecology of a suburban population of woodpigeons Columba palumbus in northwest England. Bird Study 48:361–366
Sommerfeld J, Kato A, Ropert-Coudert Y, Garthe S, Hindell MA (2013) The individual counts: within sex differences in foraging strategies are as important as sex-specific differences in masked boobies Sula dactylatra. J Avian Biol 44:531–540
Ste-Marie E, Grémillet D, Fort J, Patterson A, Brisson-Curadeau É, Clairbaux M, Perret S, Speakman JR, Elliott KH (2022) Accelerating animal energetics: high dive costs in a small seabird disrupt the dynamic body acceleration–energy expenditure relationship. J Exp Biol 225:jeb243252
Tian X, Gong Z, Zhang Y, Xu W, Liu H, Zhou Z, Liu D, Wang Z (2020) The impact of tag position and mass on motor behavior in pigeons. J Ornithol 161:1167–1174
Tomiałojć L (1976) The urban population of the woodpigeon Columba palumbus Linnaeus, 1758, in Europe - Its origin, increase and distribution. Acta Zool Cracov 21:587–631
Tomiałojć L (1999) A long-term study of changing predation impact on breeding woodpigeons. In: Cowand DP, Feare CJ (eds) Advances in vertebrate pest management. Filander Verlag, Fürth, pp 205–218
Tsuda Y, Kawabe R, Tanaka H, Mitsunaga Y, Hiraishi T, Yamamoto K, Nashimoto K (2006) Monitoring the spawning behaviour of chum salmon with an acceleration data logger. Ecol Freshw Fish 15:264–274
Valletta JJ, Torney C, Kings M, Thornton A, Madden J (2017) Applications of machine learning in animal behaviour studies. Anim Behav 124:203–220
Wang G (2019) Machine learning for inferring animal behavior from location and movement data. Ecol Inf 49:69–76
Whitford M, Klimley AP (2019) An overview of behavioral, physiological, and environmental sensors used in animal biotelemetry and biologging studies. Anim Biotelem 7:26
Williams HJ, Taylor LA, Benhamou S et al (2020) Optimizing the use of biologgers for movement ecology research. J Anim Ecol 89:186–206
Williams TM, Wolfe L, Davis T, Kendall T, Richter B, Wang Y, Bryce C, Elkaim GH, Wilmers CC (2014) Instantaneous energetics of puma kills reveal advantage of felid sneak attacks. Science 346:81–85
Wilson RP, Shepard ELC, Liebsch N (2008) Prying into the intimate details of animal lives: use of a daily diary on animals. Endanger Species Res 4:123–137
Witt K, Mitschke A, Luniak M (2005) A comparison of common breeding bird populations in Hamburg, Berlin and Warsaw. Acta Ornithol 40:139–146
Wittenberg J (1980) Brutzeit und zeitliche Verteilung der Bruten einer Population der Ringeltaube (Columba palumbus). J Ornithol 121:96–101
Wood SN, Pya N, Säfken B (2016) Smoothing parameter and model selection for general smooth models. J Am Stat Assoc 111:1548–1563
Yeap L, Warren KS, Bouten W, Vaughan-Higgins R, Jackson B, Riley K, Rycken S, Shephard JM (2022) Application of tri-axial accelerometer data to the interpretation of movement and behaviour of threatened black cockatoos. Wildlife Res 49:100–110
Yoda K, Naito Y, Sato K, Takahashi A, Nishikawa J, Ropert-Coudert Y, Kurita M, Le Maho Y (2001) A new technique for monitoring the behaviour of free-ranging Adelie penguins. J Exp Biol 204:685–690
Yu H, Deng J, Nathan R, Kröschel M, Pekarsky S, Li G, Klaassen M (2021) An evaluation of machine learning classifiers for next-generation, continuous-ethogram smart trackers. Mov Ecol 9:15
Zhang G, Patuwo BE, Hu MY (1998) Forecasting with artificial neural networks: the state of the art. Int J Forecast 14:35–62
Zhang VY, Williams CT, Theimer TC, Buck CL (2019) Reproductive and environmental drivers of time and activity budgets of striped skunks. Integr Org Biol 1:obz013
Zhou Q, Wei F, Huang C, Li M, Ren B, Luo B (2007) Seasonal variation in the activity patterns and time budgets of Trachypithecus francoisi in the Nonggang Nature Reserve, China. Int J Primatol 28:657–671
Zimmer I, Ropert-Coudert Y, Kato A, Ancel A, Chiaradia A (2011) Does foraging performance change with age in female little penguins (Eudyptula minor)? PLoS ONE 6:e16098
Acknowledgements
We are grateful to Sascha Rösner, Kim Lindner, Nicolas Frieß, Thomas Nauss, Martin Beal, Maria Dias, Josef Kühling, Marie Claire Gatt, Lennart Wegner, Jennifer Greiner, and Sabine Wagner for their contributions to the fieldwork, logistics, and laboratory work. We acknowledge funding from the German Ornithologists’ Society (DO-G) and the Hessian State Ministry for Higher Education, Research and the Arts, Germany, as part of the LOEWE priority project Nature 4.0—Sensing Biodiversity. Two reviewers helped to improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work of JFM was funded by the Hessian Ministry for Higher Education, Research, Science and the Arts, Germany, as part of the LOEWE priority project Nature 4.0—Sensing Biodiversity (https://www.uni-marburg.de/en/fb19/natur40). GPS-GSM/GPRS trackers were funded by the Hessian Ministry for Higher Education, Research, Science and the Arts, Germany, as part of the LOEWE priority project Nature 4.0—Sensing Biodiversity and the Justus Liebig University Giessen (to PQ), and a research grant to YRS by the German Ornithologists’ Society (DO-G).
Author information
Authors and Affiliations
Contributions
PQ and JFM conceived and designed the study. PQ acquired the necessary funding. JFM, YRS, BM, and PQ carried out the extensive fieldwork and data collection. WR wrote the R script. JFM and PQ carried out the analyses. JFM drafted the manuscript. All authors reviewed the final draught of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study involved free-ranging individuals. All applicable institutional and/or national guidelines for the care and use of animals were followed. Animal handling, including an ethical approval according to the national animal protection laws, was carried out under permits of the Regierungspräsidium Gießen (permit numbers G51/2017 and G10/2019) and the Instituto da Conservação da Natureza e das Florestas (permit numbers 933/2018/CAPT, 15/2019/CAPT, and 23/2020/CAPT).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by W. Wiltschko
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Masello, J.F., Rast, W., Schumm, Y.R. et al. Year-round behavioural time budgets of common woodpigeons inferred from acceleration data using machine learning. Behav Ecol Sociobiol 77, 40 (2023). https://doi.org/10.1007/s00265-023-03306-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03306-w