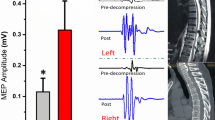
Abstract
Purpose
The aim of this study was to explore the relationship between intraoperative somatosensory evoked potential (SEP) amplitude changes and clinical outcomes of OLIF indirect decompression for degenerative lumbar spinal stenosis (DLSS).
Methods
A prospective study was performed on 201 patients who received oblique lumbar interbody fusion (OLIF) in our hospital from July 2017 to May 2021 due to single segmental DLSS. The patients were divided into three groups: group A (mild DLSS), group B (moderate DLSS), and group C (severe DLSS). The P40 amplitude during operation were recorded, and the clinical efficacy was evaluated by JOA score 1 year postoperative. ROC curves for satisfactory efficacy of P40 amplitude improvement rate and CSA improvement rate were established. Pearson correlation was used to analyze the relationship between P40 improvement rate and JOA improvement rate.
Results
In group A and group B, the improvement rate of JOA in P40 significantly improved group was significantly greater that in improved group and unimproved group (Pa = 0.009; Pb < 0.000). No significant among-subgroup differences in group C (all P > 0.05). In both groups A and B, there was a significant difference in the improvement rate of P40 amplitude between the satisfactory group and the ineffective group (Pa = 0.013; Pb = 0.001), while in group C, there was no statistical significance (Pc = 0.107). By variable Person correlation analysis, a significant positive correlation was obtained between JOA improvement rate and P40 amplitude improvement rate in groups A and B (r1 = 0.27, P1 = 0.02; r2 = 0.508, P2 = 0.001), no correlation between the two in group C (r3 = 0.243, P3 = 0.056). The area under the ROC for assessing surgical efficacy in terms of CSA improvement rate was 0.813 (95% CI: 0.737–0.889, P < 0.001) and 0.767 (95% CI: 0.677–0.856, P < 0.001) in group A and group B, respectively, with satisfactory efficacy cutoff points of 50.18% and 67.89%.
Conclusion
For mild and moderate DLSS, the intraoperative P40 amplitude improvement rate can predict the improvement of clinical symptoms after surgery and can be used as a reference index to assess the effect of indirect decompression. For severe DLSS, the P40 amplitude improvement rate has limited significance in guiding indirect decompression, and OLIF indirect decompression is not the right treatment for this type of patients.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Liu L, Dong J, Wang D, Zhang C, Zhou Y (2022) Clinical outcomes and quality of life in elderly patients treated with a newly designed double tube endoscopy for degenerative lumbar spinal stenosis. Orthop Surg 14(7):1359–1368. https://doi.org/10.1111/os.13304
Jia R, Wang XQ, Zhang Y, Hsueh S (2022) Long-term outcomes after minimally invasive bilateral or unilateral laminotomy for degenerative lumbar spinal stenosis: a minimum 10-year follow-up study. World Neurosurg 164:e1001–e1006. https://doi.org/10.1016/j.wneu.2022.05.087
Zhao XB, Ma HJ, Geng B, Zhou HG, Xia YY (2021) Early clinical evaluation of percutaneous full-endoscopic transforaminal lumbar interbody fusion with pedicle screw insertion for treating degenerative lumbar spinal stenosis. Orthop Surg 13(1):328–337. https://doi.org/10.1111/os.12900
Feng P, Kong Q, Zhang B, Liu J, Ma J, Hu Y (2022) Analysis of curative effect of percutaneous coaxial large channel endoscopic lumbar interbody fusion in the treatment of degenerative lumbar spinal stenosis. Front Surg 9:1002734. https://doi.org/10.3389/fsurg.2022.1002734
Li P, Tong Y, Chen Y, Zhang Z, Song Y (2021) Comparison of percutaneous transforaminal endoscopic decompression and short-segment fusion in the treatment of elderly degenerative lumbar scoliosis with spinal stenosis. Bmc Musculoskel Dis 22(1):906. https://doi.org/10.1186/s12891-021-04804-6
MacDonald DB, Dong C, Quatrale R, Sala F, Skinner S, Soto F, Szelenyi A (2019) Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin Neurophysiol 130(1):161–179. https://doi.org/10.1016/j.clinph.2018.10.008
Macerollo A, Brown M, Kilner JM, Chen R (2018) Neurophysiological changes measured using somatosensory evoked potentials. Trends Neurosci 41(5):294–310. https://doi.org/10.1016/j.tins.2018.02.007
Koffie RM, Morgan CD, Giraldo JP, Angel S, Walker CT, Godzik J, Catapano JS, Hemphill C, Uribe JS (2022) Should somatosensory and motor evoked potential monitoring be used routinely in all posterior cervical operations for degenerative conditions of the cervical spine? World Neurosurg 162:e86–e90. https://doi.org/10.1016/j.wneu.2022.02.080
Essa ZM, Al-Hashimi AF, Nema IS (2018) Dermatomal versus mixed somatosensory evoked potentials in the diagnosis of lumbosacral spinal canal stenosis. J Clin Neurophysiol 35(5):388–398. https://doi.org/10.1097/WNP.0000000000000491
Liu X, Konno S, Miyamoto M, Gembun Y, Horiguchi G, Ito H (2009) Clinical usefulness of assessing lumbar somatosensory evoked potentials in lumbar spinal stenosis. Clinical article J Neurosurg-Spine 11(1):71–78. https://doi.org/10.3171/2009.3.SPINE08513
Jorge A, Zhou J, Dixon EC, Hamilton KD, Balzer J, Thirumala P (2019) Area under the curve of somatosensory evoked potentials detects spinal cord injury. J Clin Neurophysiol 36(2):155–160. https://doi.org/10.1097/WNP.0000000000000563
Zhang Z, Wang Y, Luo T, Qi H, Cai L, Yuan Y, Li J (2022) Dermatomal somatosensory evoked potentials and cortical somatosensory evoked potentials assessment in congenital scoliosis. Bmc Neurol 22(1):58. https://doi.org/10.1186/s12883-022-02579-4
Lee GY, Lee JW, Choi HS, Oh KJ, Kang HS (2011) A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol 40(8):1033–1039. https://doi.org/10.1007/s00256-011-1102-x
MacDonald DB, Al ZZ, Khoudeir I, Stigsby B (2003) Monitoring scoliosis surgery with combined multiple pulse transcranial electric motor and cortical somatosensory-evoked potentials from the lower and upper extremities. Spine 28(2):194–203. https://doi.org/10.1097/00007632-200301150-00018
Charalampidis A, Jiang F, Wilson J, Badhiwala JH, Brodke DS, Fehlings MG (2020) The use of intraoperative neurophysiological monitoring in spine surgery. Glob Spine J 10(1 Suppl):104S-114S. https://doi.org/10.1177/2192568219859314
Thirumala PD, Huang J, Thiagarajan K, Cheng H, Balzer J, Crammond DJ (2016) Diagnostic accuracy of combined multimodality somatosensory evoked potential and transcranial motor evoked potential intraoperative monitoring in patients with idiopathic scoliosis. Spine 41(19):E1177–E1184. https://doi.org/10.1097/BRS.0000000000001678
Reddy RP, Chang R, Rosario BP, Sudadi S, Anetakis KM, Balzer JR, Crammond DJ, Shaw JD, Thirumala PD (2021) What is the predictive value of intraoperative somatosensory evoked potential monitoring for postoperative neurological deficit in cervical spine surgery? -a meta-analysis. Spine J 21(4):555–570. https://doi.org/10.1016/j.spinee.2021.01.010
Aalto TJ, Malmivaara A, Kovacs F, Herno A, Alen M, Salmi L, Kroger H, Andrade J, Jimenez R, Tapaninaho A, Turunen V, Savolainen S, Airaksinen O (2006) Preoperative predictors for postoperative clinical outcome in lumbar spinal stenosis: systematic review. Spine 31(18):E648–E663. https://doi.org/10.1097/01.brs.0000231727.88477.da
Sanchez RM, Mora GF, Oflidis V, Margetis K, Tellez MJ, Ulkatan S, Kimura J (2022) Optimizing the methodology for saphenous nerve somatosensory evoked potentials for monitoring upper lumbar roots and femoral nerve during lumbar spine surgery: technical note. J Clin Monit Comput 36(4):1079–1085. https://doi.org/10.1007/s10877-021-00737-6
Owen JH, Padberg AM, Spahr-Holland L, Bridwell KH, Keppler L, Steffee AD (1991) Clinical correlation between degenerative spine disease and dermatomal somatosensory-evoked potentials in humans. Spine 16(6 Suppl):S201–S205. https://doi.org/10.1097/00007632-199106001-00005
Cohen BA, Major MR, Huizenga BA (1991) Predictability of adequacy of spinal root decompression using evoked potentials. Spine 16(8 Suppl):S379–S384. https://doi.org/10.1097/00007632-199709010-00005
Storm SA, Kraft GH (2004) The clinical use of dermatomal somatosensory evoked potentials in lumbosacral spinal stenosis. Phys Med Reh Clin N 15(1):107–115. https://doi.org/10.1016/s1047-9651(03)00107-4
Zhu YL, Xie ZL, Wu YW, Duan WR, Xie YK (2012) Early demyelination of primary A-fibers induces a rapid-onset of neuropathic pain in rat. Neuroscience 200:186–198. https://doi.org/10.1016/j.neuroscience.2011.10.037
Cheng XH, Zhang L, Fu J (2019) Somatosensory evoked potential changes and decompression timing for spinal cord function recovery and evoked potentials in rats with spinal cord injury. Brain Res Bull 146:7–11. https://doi.org/10.1016/j.brainresbull.2018.12.003
Huang S, Garstka ME, Murcy MA, Bamford JA, Kang SW, Randolph GW, Kandil E (2019) Somatosensory evoked potential: preventing brachial plexus injury in transaxillary robotic surgery. Laryngoscope 129(11):2663–2668. https://doi.org/10.1002/lary.27611
Tsai SW, Tsai CL, Wu PT, Wu CY, Liu CL, Jou IM (2012) Intraoperative use of somatosensory-evoked potential in monitoring nerve roots. J Clin Neurophysiol 29(2):110–117. https://doi.org/10.1097/WNP.0b013e31824cecd3
Osenbach RK, Hitchon PW, Mouw L, Yamada T (1993) Effects of spinal cord ischemia on evoked potential recovery and postischemic regional spinal cord blood flow. J Spinal Disord 6(2):146–154. https://doi.org/10.1097/00024720-199304000-00009
Acknowledgements
The authors would like to thank all study participants who were enrolled in this study.
Funding
This study was supported by Ningxia Provincial Key Research and Development Planed Projects (No. 2020BEG03034).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Zhiqiang Wang and Shulong Yang contributed equally to this work. Material preparation, data collection, and analysis were performed by Dr Zhiqiang Wang and Dr Shulong Yang. The first draft of the manuscript was written by Dr Zhiqiang Wang and Dr Shulong Yang, and critical revision was done by Dr Simin Liang, Wanzhong Yang, Anli Shi, Wei Guo, and Wei Yang. Project administration was carried out by Dr Zhaohui Ge. All the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of the General of Ningxia Medical University (No. 2019–38) and performed according to the tenets of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all enrolled patients.
Consent for publication
The authors agree to publication. This manuscript has not been published in any journals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Yang, S., Liang, S. et al. The value of somatosensory evoked potentials in intraoperative evaluation of indirect decompression effect of oblique lumbar interbody fusion for lumbar spinal stenosis. International Orthopaedics (SICOT) 47, 2055–2064 (2023). https://doi.org/10.1007/s00264-023-05790-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-023-05790-1