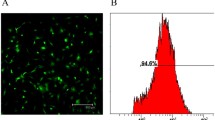
Abstract
Purpose
The myofibroblast, a contractile fibroblastic cell expressing α-smooth muscle actin (α-SMA), has been reported to play a role in ligament healing. The aim of this study was to evaluate the feasibility of transplanting culture-derived myofibroblasts in injured rabbit medial collateral ligaments (MCL) and in intact anterior cruciate ligaments (ACL).
Methods
Fibroblasts isolated from the iliotibial band were cultured in the presence of transforming growth factor beta-1 (TGF-β1) for five days and analysed for α-SMA expression. In a concentration of TGF-β1 ≥ 10 ng/ml, the differentiation rate into myofibroblast was 90%. After labelling with PKH26, α-SMA -positive cells were transplanted in intact ACL and in injured MCL of ten rabbits.
Results
Survival of PKH-26+ cells was seen in all intact and damaged ligaments one day after injection. The density of PKH-26+ cells had decreased at seven days postinjection in both ligaments. Double-positive PKH-26+/α-SMA+ cells were only observed in injured MCL at seven days postinjection. Moreover, we found that genetically modified fibroblasts differentiate into myofibroblasts and can be transplanted into ligaments.
Conclusions
Our data demonstrate that culture-born myofibroblasts survive and maintain α-SMA expression up to one week after transplantation. This study provides the first insight into the feasibility of transplanted mechanically active cells for ligament reconstruction.



Similar content being viewed by others
References
Woo SL, Abramowitch SD, Kilger R, Liang R (2006) Biomechanics of knee ligaments: injury, healing, and repair. J Biomech 39(1):1–20
Woo SL, Vogrin TM, Abramowitch SD (2000) Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg 8(6):364–372
Frank CB (1996) Ligament healing: current knowledge and clinical applications. J Am Acad Orthop Surg 4(1):74–83
Weiss JA, Woo SL, Ohland KJ, Horibe S, Newton PO (1991) Evaluation of a new injury model to study medial collateral ligament healing: primary repair versus nonoperative treatment. J Orthop Res 9(4):516–528
Sanchez M, Anitua E, Lopez-Vidriero E, Andia I (2010) The future: optimizing the healing environment in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev 18(1):48–53
Day CS, Kasemkijwattana C, Menetrey J, Floyd SS Jr, Booth D, Moreland MS, Fu FH, Huard J (1997) Myoblast-mediated gene transfer to the joint. J Orthop Res 15(6):894–903
Hildebrand KA, Deie M, Allen CR, Smith DW, Georgescu HI, Evans CH, Robbins PD, Woo SL (1999) Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: the use of different viral vectors and the effects of injury. J Orthop Res 17(1):37–42
Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Fu FH, Moreland MS, Huard J (1999) Direct-, fibroblast- and myoblast-mediated gene transfer to the anterior cruciate ligament. Tissue Eng 5(5):435–442
Wang CJ, Weng LH, Hsu SL, Sun YC, Yang YJ, Chan YS, Yang YL (2010) pCMV-BMP-2-transfected cell-mediated gene therapy in anterior cruciate ligament reconstruction in rabbits. Arthroscopy 26(7):968–976
Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, Kubo S, Kawamoto A, Asahara T, Kurosaka M, Kuroda R (2008) Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem cells 26(3):819–830
Yasuda K, Tomita F, Yamazaki S, Minami A, Tohyama H (2004) The effect of growth factors on biomechanical properties of the bone-patellar tendon-bone graft after anterior cruciate ligament reconstruction: a canine model study. Am J Sports Med 32(4):870–880
Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, Schmidt CC (1998) The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med 26(4):549–554
Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM (2009) Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med 37(12):2401–2410
Ivkovic A, Marijanovic I, Hudetz D, Porter RM, Pecina M, Evans CH (2011) Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 3:923–944
Darby I, Skalli O, Gabbiani G (1990) Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63(1):21–29
Schurch W, Seemayer TA, Gabbiani G (1998) The myofibroblast: a quarter century after its discovery. Am J Surg Pathol 22(2):141–147
Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C (1977) Ultrastructural and immunochemical evidence of actin in the tendon cells. Clin Orthop Relat Res 126:282–284
Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C (1980) Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am 62(4):583–598
Postacchini F, Natali PG, Accinni L, Ippolito E, de Martino C (1977) Contractile filaments in cells of regenerating tendon. Experientia 33(7):957–959
Murray MM, Martin SD, Martin TL, Spector M (2000) Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am 82-A(10):1387–1397
Murray MM, Spector M (1999) Fibroblast distribution in the anteromedial bundle of the human anterior cruciate ligament: the presence of alpha-smooth muscle actin-positive cells. J Orthop Res 17(1):18–27
Weiler A, Unterhauser FN, Bail HJ, Huning M, Haas NP (2002) Alpha-smooth muscle actin is expressed by fibroblastic cells of the ovine anterior cruciate ligament and its free tendon graft during remodeling. J Orthop Res 20(2):310–317
Faryniarz DA, Chaponnier C, Gabbiani G, Yannas IV, Spector M (1996) Myofibroblasts in the healing lapine medial collateral ligament: possible mechanisms of contraction. J Orthop Res 14(2):228–237
Menetrey J, Laumonier T, Garavaglia G, Hoffmeyer P, Fritschy D, Gabbiani G, Bochaton-Piallat ML (2011) alpha-Smooth muscle actin and TGF-beta receptor I expression in the healing rabbit medial collateral and anterior cruciate ligaments. Injury 42(8):735–741
Salmon P, Kindler V, Ducrey O, Chapuis B, Zubler RH, Trono D (2000) High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood 96(10):3392–3398
Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB (2007) Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res 25(8):1007–1017
Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G (1986) A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103(6 Pt 2):2787–2796
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122(1):103–111
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3(5):349–363
Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ (2007) Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19(4):761–771
Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G (2001) Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159(3):1009–1020
DesRosiers EA, Yahia L, Rivard CH (1996) Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J Orthop Res 14(2):200–208
Marui T, Niyibizi C, Georgescu HI, Cao M, Kavalkovich KW, Levine RE, Woo SL (1997) Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res 15(1):18–23
Acknowledgments
This work was supported by the Swiss National Science Foundation (NRP 46 n°4046-058639 and grant n°310030_130700/1), by the Fondation suisse de recherche sur les maladies musculaires, by the Research funds of the Geneva Orthopedic Service and by the Foundation Marcel Levaillant.
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laumonier, T., Michel, M., Gabbiani, G. et al. Autologous transplantation of culture-born myofibroblasts into intact and injured rabbit ligaments. International Orthopaedics (SICOT) 36, 1733–1738 (2012). https://doi.org/10.1007/s00264-012-1519-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-012-1519-4