Abstract
Background
Advancements in immunotherapeutic approaches only had a modest impact on the therapy of lung neuroendocrine neoplasms (LNENs). Our multicenter study aimed to investigate the expression patterns of novel immunotherapy targets in intermediate- and high-grade LNENs.
Methods
The expressions of V-domain Ig suppressor of T cell activation (VISTA), OX40L, Glucocorticoid-induced TNF receptor (GITR), and T cell immunoglobulin and mucin domain 3 (TIM3) proteins were measured by immunohistochemistry in surgically resected tumor samples of 26 atypical carcinoid (AC), 49 large cell neuroendocrine lung cancer (LCNEC), and 66 small cell lung cancer (SCLC) patients. Tumor and immune cells were separately scored.
Results
Tumor cell TIM3 expression was the highest in ACs (p < 0.001), whereas elevated tumor cell GITR levels were characteristic for both ACs and SCLCs (p < 0.001 and p = 0.011, respectively). OX40L expression of tumor cells was considerably lower in ACs (vs. SCLCs; p < 0.001). Tumor cell VISTA expression was consistently low in LNENs, with no significant differences across histological subtypes. ACs were the least immunogenic tumors concerning immune cell abundance (p < 0.001). Immune cell VISTA and GITR expressions were also significantly lower in these intermediate-grade malignancies than in SCLCs or in LCNECs. Immune cell TIM3 and GITR expressions were associated with borderline prognostic significance in our multivariate model (p = 0.057 and p = 0.071, respectively).
Conclusions
LNEN subtypes have characteristic and widely divergent VISTA, OX40L, GITR, and TIM3 protein expressions. By shedding light on the different expression patterns of these immunotherapy targets, the current multicenter study provides support for the future implementation of novel immunotherapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung neuroendocrine neoplasms (LNENs) account for one fifth of all pulmonary malignancies and comprise four histological subtypes with different clinical and biological characteristics [1,2,3,4,5]. Pulmonary carcinoids represent 10% of LNENs and comprise two major subtypes: typical and atypical carcinoids. Typical carcinoids are highly differentiated tumors that are predominantly curable by surgical resection. Accordingly, the five-year overall survival (OS) rate of these patients exceeds 80% and the recurrence rate is low [6]. Meanwhile, atypical carcinoids (ACs) are moderately differentiated, intermediate-grade tumors with greater metastatic potential compared to typical carcinoids, and with a five-year OS rate of 50%. As a result of their aggressive nature, ACs often necessitate adjuvant chemotherapy after surgical resection [7,8,9]. Large cell neuroendocrine lung cancer (LCNEC) is a poorly differentiated, high-grade tumor with a complex biology that shares similarities with both small cell lung cancer (SCLC) and non-SCLC (NSCLC). Therapeutic approaches often overlap with management protocols for SCLC, the most lethal lung carcinoma [10]. SCLC is a heterogeneous malignancy characterized by genomic instability, early metastasis, and a rapid proliferation rate. As this tumor type is often diagnosed at an advanced stage, curative-intent surgery is rarely performed, and treatment usually consists of chemo-immunotherapy with or without radiation [11, 12].
Targeted therapy and immunotherapy have revolutionized the management protocols of NSCLC patients, yet the therapeutic advancements in LNENs are poor [13,14,15,16,17]. This is primarily due to the lack of targetable driver mutations and to the conflicting results of immunotherapy-related trials [12]. The tumor immune microenvironment (TIM) has been shown to play a key role in the efficacy of immune checkpoint inhibitors (ICIs) [18, 19]. In this context, assessment of TIM, characteristic immune checkpoints, and specific immune patterns of LNENs is an essential step in understanding and improving the efficacy of currently used and forthcoming immunotherapeutic approaches.
In addition to the well-studied immune checkpoint molecules such as programmed cell death protein 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4), other molecules with relevant functions in antitumor immunity are worth investigating [20,21,22,23]. One of these molecules of potential clinical importance is the V-domain Ig suppressor of T cell activation (VISTA), a transmembrane protein that inhibits the effector function of T cells. VISTA is usually highly expressed in tumor-infiltrating lymphocytes, leading to a decreased antitumoral immune response [24]. High VISTA expression has been described in various malignancies such as melanoma, NSCLC, and pleural mesothelioma [25,26,27]. OX40L (CD252) is the ligand of the OX40 (CD134) receptor and is usually expressed by antigen-presenting cells (APCs) such as dendritic cells or macrophages [28]. Importantly, some studies have highlighted that agonists of OX40 and OX40L can enhance antitumoral immunity [29]. Glucocorticoid-induced TNF receptor (GITR) is also a transmembrane protein and plays a pivotal role in the regulation of effector T cells. Importantly, its activation can regulate antitumoral immune response [30, 31]. T cell immunoglobulin and mucin domain 3 (TIM3) is an immunoregulatory protein of T lymphocytes, myeloid cells, and several tumor cells (TCs) (e.g., melanoma, breast, and kidney cancer). Since TIM3 promotes the development of several tumors by suppressing antitumoral immunity, blockage of the TIM3 pathway might be a promising therapeutic approach [32, 33]. Although the druggability of these novel immunotherapy targets has already been validated in preclinical settings, their therapeutic relevance has not yet been assessed in LNEN patients [28, 34,35,36,37]. Nevertheless, elucidating their expression pattern in human LNENs should be among the first steps in planning specific clinical trials evaluating the efficacy of particular immunotherapeutics directed against VISTA, OX40L, GITR, and TIM3.
In order to provide insights into the applicability of immunotherapy in highly malignant LNENs, the current study aimed to investigate the expression levels and distribution patterns of immunologic markers of potential therapeutic relevance in SCLC, AC, and LCNEC patients. Notably, to provide a comprehensive overview of immunologic marker expression within the whole tumor, the study was conducted using surgically resected specimens.
Methods
Study population and treatment
A total of 141 surgically treated LNEN patients from the following four Central European centers were included: National Koranyi Institute of Pulmonology (Budapest, Hungary), National Institute of Oncology (Budapest, Hungary), Medical University of Graz (Graz, Austria), and Palacky University Olomouc (Olomouc, Czech Republic). Of these patients, 66, 49, and 26 were diagnosed with SCLC, LCNEC, and AC, respectively. Concerning the enrollment period, SCLC samples originated from between 1997 and 2020, whereas LCNEC and AC FFPE blocks were all created in 2016–2021 and 2008–2019, respectively. Only whole-tissue specimens were included to avoid bias due to intratumoral heterogeneity. To achieve a representative cohort size, all individuals who underwent surgical resection for LNEN in the participating institutions were included. Further inclusion criteria consisted of adequate tumor content (> 20% of all cells) in FFPE blocks as defined by an expert pathologist. Clinicopathological data were collected retrospectively from the medical records of each center. The study was conducted in accordance with the guidelines of the Helsinki Declaration of the World Medical Association and with the approval of the national-level Ethics Committee of each participating country. Patient identifiers were removed after clinical data collection to ensure patient pseudonymity. The requirement for written informed consent was waived due to the study’s retrospective nature. All patients underwent lung resection surgery, and adjuvant chemo- and/or radiotherapy was administered when necessary. Each therapeutic approach was applied in accordance with the contemporary National Comprehensive Cancer Network (NCCN) guidelines.
Patient samples and immunohistochemistry
Prior to enrolment, all slides were re-evaluated by a board-certified pathologist to confirm the diagnosis of LNEN. Next, tissue samples were analyzed for the expression of the four TIM markers including TIM3, VISTA, GITR, and OX40L. Due to the low tissue quantity of samples, in 21 SCLC cases, only VISTA expression was measured. The specific antibodies against these markers are summarized in Supplementary Table S1. To assess the quality and reliability of the older (> 15 years) FFPE blocks concerning SCLC patients, these samples were also stained with routinely used diagnostic antibodies against CD56 [38] and Ki-67 [39]. The degree of immune infiltration was assessed by analyzing CD3 expression. Immunohistochemistry (IHC) staining was performed according to the recommended staining protocols using Ventana BenchMark Ultra IHC/ISH System. (Roche diagnostics, Basel, Switzerland). In brief, after deparaffinization and incubation with the primary antibody, the secondary antibody was applied for one hour at room temperature. Visualization of the expression levels was achieved with Liquid DAB and Substrate Chromogen System, and sections were counterstained with hematoxylin. Of note, the staining protocol was validated by human tonsils as positive tissue controls. Expression of the given marker was examined blinded to clinical data by two experienced independent lung pathologists. All slides were digitally scanned using PANNORAMIC 250 Flash III (3DHISTECH Ltd., Budapest, Hungary); sections were examined and evaluated by using CaseViewer 2.4 (3DHISTECH Ltd., Budapest, Hungary). Providing an overview of generalized marker expression across the tumor area is essential for biomarker discovery[40]. Therefore, during pathological evaluation, we determined the percentage of positive TCs in at least 20 randomly selected areas at 20 × and 40 × magnification. Two experienced pulmonary pathologists performed the evaluation process, and if a discrepancy of > 20% occurred in their results, a third pulmonary pathologist was also involved.
TCs were evaluated separately from immune cells (ICs). In the case of TCs, the ratio of positive cells to all TCs was also quantified. Similarly, the ratio of ICs showing positive staining and the ratio of total immune infiltrates in a given sample were determined. It should be emphasized that manual analysis of each marker was preferred in this study since software-based evaluation still bears many limitations, even for antibodies used in routine diagnostics.
Statistical analysis
All statistical analyses were performed in R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Associations between histological subtypes and clinicopathological characteristics were assessed by Fisher’s exact tests and Kruskal–Wallis rank sum tests for categorical and continuous variables, respectively. Adjustment for multiple comparisons was achieved with the Bonferroni-method. Marker expression levels and clinicopathological parameters were compared with Wilcoxon signed-rank tests with Bonferroni correction. Hierarchical clustering of samples based on the expression levels was performed with the ComplexHeatmap R package (version 2.10.0). The distance matrix was calculated using Manhattan distance measure and the dendrograms were created using the ward.D clustering method. Pearson-correlation coefficients (R) were calculated between expression levels, with p values corrected for multiple comparisons with the Bonferroni-method. To investigate which expression levels are most indicative of LNEN subtype, a principal component analysis (PCA) was performed (with the factoextra R package (version 1.0.7)) to find linear combinations (“principal components” (PCs)) of the measured variables (expression levels) that most effectively explain the variance in the data. Clinical factors having a prognostic relevance for OS were determined by univariate Kaplan–Meier analysis. Survival curves of different patient subgroups were compared with log-rank tests. P values were not adjusted for multiple testing. To assess the prognostic relevance of different marker expression, patients were divided into “low” (i.e., median or below-median) vs. “high” (i.e., above-median) expressing groups based on the median expression value of each marker. Guided by the results of univariate analyses, a multivariate Cox-regression model was fitted to the data.
Results
Patient and sample characteristics
Clinicopathological features of included patients according to LNEN histological subtypes are summarized in Table 1. Most SCLC and LCNEC patients were smokers, whereas the majority of individuals diagnosed with AC were never-smokers (p < 0.001). SCLC tumors tended to be centrally located, contrasting the peripheral localization of LCNEC (p < 0.001). Univariate models were applied to evaluate survival outcomes considering clinicopathological characteristics. Diabetes (p = 0.019), histological subtype (p = 0.035), vascular involvement (p = 0.0063), as well as T and N stages (p = 0.078 and 0.044, respectively) were all relevant factors for survival (Supplementary Fig. 1). As for the antibodies used for quality check of the older (> 15 years) FFPE samples, we found strong positivity with CD56 and moderate positivity (associated with reduction of immunosignal intensity) with Ki-67 (Supplementary Fig. 2). Notably, expression patterns of TIM3, VISTA, GITR, and OX40L did not differ statistically significantly between the older (> 15 years) and newer (≤ 15 years) blocks.
Distribution pattern of immunologic markers by TCs
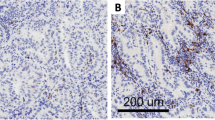
Representative IHC images of each investigated marker and their TC expression levels according to LNEN subtypes are shown in Figs. 1 and 2A, respectively. Interestingly, OX40L expression of AC TCs was significantly lower than in SCLC tumors (p < 0.001). Meanwhile, ACs tended to demonstrate significantly higher TC GITR expression levels than SCLC or LCNEC tumors (p < 0.001). Of note, TC GITR expression was also considerably higher in SCLC than in LCNEC (p = 0.011). As for TIM3, its TC expression was significantly higher in ACs (vs. LCNEC and SCLC tumors; p = 0.047 and p < 0.001, respectively). No significant differences were observed for VISTA expression. In order to examine whether LNEN subtypes can be distinguished solely by their TC VISTA, GITR, OX40L, or TIM3 expression, we performed unsupervised hierarchical clustering. As shown in Fig. 3A, although cluster analysis differentiated three distinct subgroups with divergent immunologic phenotypes, these clusters did not conclude with the histological subtypes. When examining the clinicopathological relevance of TC marker expression, we found that grade two tumors tended to have higher GITR (p = 0.028) and TIM3 (p = 0.03) expression than grade 3 lesions (Supplementary Fig. 3A). GITR expression by TCs was also significantly higher in never-smokers than in current smokers (p = 0.046); yet, this is likely to be attributed to the distinct smoking habits of each LNEN patient.
Immunohistochemistry staining of formalin-fixed, paraffin-embedded AC, LCNEC, and SCLC samples with the four immune-related markers. Representative images for TCs with positive staining were captured with a 40 × objective lens. Positive cells were visualized with 3–3’-diaminobenzidine (DAB), and nuclei were labeled with hematoxylin. Scale bar: 50 μm. AC, atypical carcinoid; LCNEC, large cell neuroendocrine lung cancer; SCLC, small cell lung cancer; and TC, tumor cell
Expression levels of potential immunotherapy targets by TCs (A) and ICs (B) in different LNEN subtypes. The color-filled curves show the estimated normalized probability density function of the data. Overlaid box plots demonstrate the same distributions, and box edges represent the first (Q1) and third (Q3) quartiles, with the inner line showing the median value. Whiskers extend to 1.5-times the interquartile range (IQR = Q3-Q1). Samples outside this range (outliers) are marked by black dots. Only significant p values are shown. Colors indicate the three LNEN subtypes, Green: AC, atypical carcinoid; yellow: LCNEC, large cell neuroendocrine lung cancer; and orange: SCLC, small cell lung cancer
Hierarchical clustering of LNEN subtypes based on TC (A) and IC (B) VISTA, OX40L, GITR, and TIM3 expression. The color bar scale indicates the expression levels of the selected markers. LCNEC, large cell neuroendocrine lung cancer; SCLC, small cell lung cancer; AC, atypical carcinoid. C Heatmap of marker expression levels as defined by both TCs and ICs. D Heatmap of the average TC and IC marker expression levels for each subtype
Distribution pattern of immunologic markers by ICs
To gain insights into the overall immune landscape of LNEN subtypes, we first assessed each tumor’s CD3 expression. We found that IC abundance was similar in LCNEC and SCLC samples but significantly lower in AC tumors (p < 0.001). ACs also expressed significantly lower levels of IC VISTA (p < 0.001) and GITR (p = 0.002) than LCNEC or SCLC tumors (Fig. 2B). Meanwhile, TIM3 expression by ICs was significantly lower in SCLCs compared to ACs (p < 0.001) or LCNECs (p < 0.001). Cluster analysis was not able to distinguish LNEN subtypes based solely on VISTA, GITR, OX40L, or TIM3 expression by ICs (Fig. 3B). With regard to clinicopathological features (Supplementary Fig. 3B), centrally located tumors tended to have significantly lower levels of immune infiltration (p < 0.001), and their ICs expressed significantly lower levels of VISTA and TIM3 than peripheral tumors (p < 0.001). Moreover, we also found that necrotic tumors presented with a significantly greater amount of immune infiltration than non-necrotic lesions (p = 0.027). Tumors with high peritumoral inflammation displayed increased immune infiltration compared to tumors with medium or low levels of peritumoral inflammation.
Correlation between the expression patterns of immune-related markers defined by TCs and ICs
As shown in Supplementary Fig. 4, TC OX40L and TIM3 expression correlated with VISTA expression (R = 0.4928, p < 0.0001 and R = 0.3083, p = 0.0245, respectively). A positive linear correlation was also found between TC TIM3 and GITR expressions (R = 0.4658, p < 0.0001). We found that the IC GITR expression correlated with both TC GITR expression and IC OX40L expression (R = 0.5416, p = 0.0233 and R = 0.5678, p = 0.011, respectively).
To assess the impact of VISTA, OX40L, GITR, and TIM3 expressions on CD3 distribution, we correlated IC CD3 expression with both IC and TC expressions of the markers above. As shown in Supplementary Fig. 5, none of the examined immunotherapy targets correlated significantly with CD3 expression; yet a tendency toward positive linear correlation could be observed in the case of IC GITR and VISTA expressions.
LNEN subtype-specific immunologic landscape revealed by principal component analysis
Principal component analysis showed that 72% of the variance in the data is explained by the first three PCs. Upon further investigation, PC1 did not seem to effectively separate patients based on LNEN subtype, and thus, we projected all data points and original variables into the space spanned by PC2 and PC3 (Fig. 4). The figure displays that by using PCA, ACs can be distinguished from both LCNEC and SCLC tumors based on their IC and TC marker expression. In this context, (1) ACs express high levels of TC TIM3 and GITR, and low levels of IC GITR; (2) both TCs and ICs of SCLCs express high levels of GITR, and their ICs express low levels of TIM3; and (3) ICs of LCNECs express high levels of GITR and TIM3. The trends for expression levels of each sample, grouped by their histologic subtype, are shown in Fig. 3C. Figure 3D highlights the average expression pattern for each type, underlining previous observations of the most typical features.
Principal component analysis of LNEN marker expression. ACs can be distinguished from LCNEC and SCLC tumors based on their IC and TC marker expression. ACs express high TC TIM3 and GITR levels and low IC GITR levels. TCs and ICs of SCLC lesions express high levels of GITR, and their ICs express low levels of TIM3. Percentage values on axis labels indicate the percentage of explained variance by the given principal component. ICs of LCNEC tumors express high levels of GITR and TIM3. AC, atypical carcinoid; LCNEC, large cell neuroendocrine lung cancer; SCLC, small cell lung cancer; and PC, principal component
Overall immunological phenotype distinguishes LNEN tumors
In our previous study [24], we investigated the expression pattern of 15 immune-related markers (PD-1, CD27, CD4, CD47, ICOS, LAG3, OX40, PD-L1, IDO, CD70, CD137, CD3, CD40, NKG2A, CD8) using a representative number of AC, LCNEC, and SCLC samples. Since 69 cases of the prior patient cohort overlapped with the cohort for this study, the datasets were merged in order to examine whether the overall marker expression distinguishes LNEN subtypes. Unsupervised clustering revealed unique marker expression patterns in the different histological samples. Importantly, the results demonstrate that the applied immune-related markers are highly effective in classifying tumors into their respective subgroups (Fig. 5A). Figure 5B demonstrates the average expression patterns of examined markers with regard to LNEN subtypes.
A Hierarchical clustering of LNENs based on the TC expression of immune-related markers. The color bar scale indicates the expression levels of CD137, CD27, CD3, CD4, CD40, CD47, CD70, CD8, GITR, ICOS, IDO, LAG3, NKG2A, OX40, OX40L, PD-1, PD-L1, TIM3, VISTA. LCNEC, large cell neuroendocrine carcinoma; SCLC, small cell lung cancer; AC, atypical carcinoid B Heatmap of the average TC expression levels of the selected immune-related markers
Association between immune marker expression levels and survival
Patients were divided into low- and high-expressing categories based on the median expression levels of the examined four immune-related markers. Although TC marker expressions did not influence the OS significantly, patients with high TC TIM3 expression tended to have improved survival outcomes compared to those with low TC TIM3 levels (p = 0.08) (Supplementary Fig. 6A). Infiltrating ICs did not have a significant prognostic implication either (Supplementary Fig. 6B). As for IC marker expression, we found that high TIM3 expression was associated with significantly improved survival outcomes (vs. low TIM3 expression, p = 0.021). Meanwhile, low IC GITR expression corresponded to an increased OS with a borderline significant trend (p = 0.064). Lastly, we performed multivariate Cox-regression analysis to decipher the clinical parameters influencing OS (Supplementary Fig. 7). Of all examined parameters, diabetes (p = 0.003), histological type (AC vs. LCNEC, p = 0.061 and AC vs. SCLC, p = 0.016), and tumor grade (p = 0.045) influenced the OS independently. Of note, the independent prognostic relevance of IC TIM3 and GITR expression remained borderline significant (p = 0.057 and p = 0.071, respectively).
Discussion
The last twenty years of lung cancer research have clarified that the immune infiltration of the tumorous and peritumoral regions significantly influences the fate of malignant lesions [41, 42]. Hence, gaining a better understanding of the TIM and the interactions between tumors and ICs is essential for improving the efficacy of targeted therapies and immunotherapy. As for the latter, the recent advances in immunotherapy in other tumor entities have not yet provided an equal breakthrough in LNENs [43]. A comprehensive understanding of the TIM is, therefore, crucial for efficacious treatment protocols in the future [44, 45]. Here, we investigated the expression pattern and clinicopathological relevance of four novel immunotherapy targets in surgically resected LNENs.
Currently, only a few predictive and prognostic markers have been identified in neuroendocrine neoplasms. Orthopedia homeobox protein (OTP) is a promising marker for pulmonary carcinoids. Indeed, OTP and the adhesion molecule CD44 have been described as potential prognostic markers; nevertheless, their exact impact on OS and immunotherapeutic efficacy is still controversial [46,47,48]. In contrast, mutation in the MEN1 (multiple endocrine neoplasia 1) gene has clearly been shown to be a poor prognostic factor in pulmonary carcinoids [49]. LCNEC has recently been classified into two major groups based on genomic and transcriptomic levels. LCNEC I is characterized by mutations in retinoblastoma protein 1 (Rb1) and tumor protein 53 (TP53), similar to SCLC. In contrast, LCNEC II exhibits alterations in NSCLC-type serine/threonine kinase 11 (STK11), Kelch-like ECH-associated protein 1 (KEAP1), and Kirsten rat sarcoma virus (KRAS); these are commonly seen in lung adenocarcinomas too. These molecular findings support the theory that LCNEC is a “mixed basket” of tumors with different origins [50,51,52]. SCLC is a particularly aggressive entity and was long considered a homogenous tumor. Recent data, however, demonstrated that SCLC tumors could be sub-segmented into distinct molecular subtypes based on the expression pattern of specific transcription factors (achaete-scute homologue 1 (ASCL1), neurogenic differentiation factor 1 (NEUROD1), POU Class 2 homeobox 3 (POU2F3)) and inflammatory characteristics. These subtypes are characterized by varying morphology, therapeutic responsiveness, and prognosis [12, 53,54,55].
VISTA is a membrane protein, expressed usually by myeloid cells, granulocytes, and T cells, and it functions as a negative checkpoint ligand for antigen-presenting cells and T cells [56]. Previous studies concerning other malignancies (e.g., lung, kidney, colorectal, endometrial and ovarian cancers) have described VISTA to be expressed by lymphocytes in the tumor microenvironment as well as by the TCs [57, 58]. Its prognostic significance is rather controversial, since high VISTA expression is associated with improved OS in epithelioid mesothelioma, but with worse survival outcomes in colorectal tumors [59,60,61]. In the present study, VISTA expression did not have a significant impact on OS. However, ICs in LCNECs and SCLCs expressed VISTA to a greater extent than in AC tumors. We postulate that VISTA might be highly expressed in malignant lesions as it inhibits T lymphocyte function, and therefore, reduces the antitumor response. Thus, VISTA might be an effective treatment target in these highly malignant lesions. In fact, experiments in murine models have confirmed that VISTA inhibition increases the number of T lymphocytes and boosts their function in the tumor environment [56]. A phase 1 clinical trial is currently investigating the efficacy of an anti-VISTA monoclonal antibody (JNJ-61610588; NCT02671955) in various solid tumors. Meanwhile, another ongoing multicenter study examines the long-term effects of CA-170, a PD-L1/PD-L2 and VISTA inhibition in solid tumors and lymphomas [56, 62,63,64,65].
The OX40 ligand “OX40L” is an immune checkpoint modulator primarily expressed on activated APCs, dendritic cells, B cells, and macrophages. [66] The attachment of OX40 and OX40L enhances the survival of CD4+ and CD8+ cells which increases tumor-specific responses of effector T cells in tumors and neutralizes the suppressive effects of Treg cells. [67] A recent study on NSCLC found that elevated OX40L expression is associated with higher CD4+ infiltration and increased OS. [68] Studies in SCLC, melanoma, and pancreatic ductal adenocarcinoma have yielded similar results. [69, 70] Our study did not show any significant survival benefit concerning OX40L expression, which might partly be explained by the low number of included cases. However, we found that AC TCs typically expressed OX40L at lower levels than other LNEN TCs. Interestingly, in our previous study [24] also conducted on LNENs, we did not find significant differences in OX40 expression concerning histological subtypes [24]. This might be suggestive of independent OX40 and OX40L expression patterns. Importantly, recent in vivo studies suggest that agonistic and antagonistic therapy toward OX40-OX40L interaction might be a potential therapeutic option [28, 66, 67].
Another attractive target for immunotherapy is GITR, a costimulatory cell surface receptor [71]. It is expressed mainly on T cells and NK cells and plays a major role in the activation of effector T cells, thereby making it an appealing target for antitumoral therapy. Indeed, triggering GITR enhances the immune system’s antitumoral response in murine models by stimulating T lymphocyte activity and inhibiting Treg cells. The first clinical trial in which the GITR agonist TRX518 was applied in solid tumors commenced in 2018. Unfortunately, the survival benefits were modest, and the primary endpoints were not met despite combining TRX518 with PD-1 and PD-L1 inhibitors [30, 31, 72, 73]. Nevertheless, several additional studies investigating GITR targeting are still ongoing [74, 75]. Increased GITR expression has been shown to be a positive prognostic factor in endometrial carcinoma and head and neck tumors, whereas the opposite has been confirmed in renal carcinoma [72, 73, 75]. In our cohort, GITR was expressed to a greater degree by AC TCs than LCNEC and SCLC TCs, and ICs in ACs expressed significantly less GITR compared to LCNEC and SCLC samples. Of note, the effects of GITR expression on tumor-infiltrating ICs also vary depending on the elapsed time. Specifically, while GITR activation initially inhibits the Treg cells and thus contributes to increased immune infiltration, activating GITR excerpts an opposite effect as time passes and inhibits the antitumor immune response [31, 34, 35, 76, 77]. Indeed, we also found in our previous study that AC tumors (where GITR expression is expected to be the highest) had considerably lower levels of tumor-infiltrating CD8+ and CD3+ lymphocytes than LCNEC or SCLC tumors with high GITR expression [24]. In terms of survival, low GITR expression in the tumor environment showed a borderline significant trend for improved survival; this trend remained similar in the Cox-regression model.
TIM3, a negative T cell regulator expressed mainly on NK cells and macrophages, was the last protein examined within the framework of the current study. Free TIM3 promotes T cell activation, but once activated, it inhibits the antitumor immune response, induces immunosuppression, and contributes to poor prognosis [32, 33, 36]. Therefore, TIM3 might emerge in the future as a promising target for inhibition [75]. Indeed, blockage of both TIM3 and PD-1 has been demonstrated to result tumor regression in preclinical models, and several clinical trials are currently exploring TIM3 inhibition in solid tumors [32, 33, 78, 79]. Interestingly, both TCs and ICs expressed TIM3 to a greater degree in ACs than in LCNEC and SCLC tumors. As for its clinical relevance, high TIM3 expression by TCs and ICs tended to be associated with improved survival. Nevertheless, patients with AC tumors generally had better prognosis than those with other LNENs, and TIM3 expression could not be validated as an independent prognosticator in our multivariate model. Still, its high expression in AC tumors makes TIM3 a potential subtype-specific immunotherapeutic target for AC patients.
Despite the evident differences in their expression pattern, cluster analysis of VISTA, OX40L, GITR, and TIM3 expression did not differentiate LNEN subtypes. Of note, however, when supplementing the current results with our findings from an overlapping cohort [24], it became apparent that LNEN tumors have widely different immune phenotypes. One particularity of this finding is that AC tumors are the least immunogenic entities among all investigated LNENs, albeit they have the highest TIM3 and GITR TC expression. These immune profiles can aid in diagnosing each histologic subset and predicting potential therapeutic responses to immune checkpoint blockade. Nevertheless, as highlighted before, VISTA, OX40L, GITR, and TIM3 expression regulate the tumor immune microenvironment through a series of complex and time-dependent processes [27, 28, 30,31,32,33, 35,36,37, 62, 76, 79]. These aspects should be considered when assessing their impact on intratumoral immune cell distribution and antitumor response.
Our study has some limitations that must be addressed in future prospective settings. A small fraction (4,9%) of surgically resected tissue samples were older than 15 years. While most antigens in FFPE blocks are well preserved over time [80, 81], decreasing nuclear immunosignal intensity might occur in some of these older blocks. Of note, however, during our quality check, we obtained positive staining with routine diagnostic antibodies (CD56 [38] and Ki-67[39]) even in the three oldest blocks, and we found no statically significant differences between the older and newer FFPE blocks concerning immunotherapy target expression. Another limitation was that clinical and follow-up data were not available in all cases due to the study’s retrospective nature. This hindered subsequent analyses and precluded in-depth measurements (i.e., cancer-specific survival). Albeit this lack of data did not influence our findings concerning the expression pattern of investigated markers, all results deriving from clinical data analysis warrant further validation. Furthermore, although a relatively large number of rare tumors was collected, the cohort size was still small to reach statistical significance in some instances. Lastly, the current study is rather descriptive and hypothesis-generating than evidence-based since the direct effects of immunotherapeutics could not be assessed. Accordingly, the key immunologic drivers of marker expression could not be assessed within the framework of the current study and need to be verified in future experimental settings. Of note, these limitations were partly counterbalanced because all analyses were conducted on surgically resected specimens, thus avoiding the distorting effects of intra-tumoral heterogeneity and obtaining a complete overview of the tumors’ immunologic landscape.
By investigating the expression pattern of potential immunotherapy targets in intermediate- and high-grade LNENs, the current multicenter study aimed to aid the future implementation of novel immunotherapeutic approaches. We report that high TC TIM3 expression is characteristic of AC tumors, whereas elevated TC GITR levels could be found in both ACs and SCLCs. OX40L expression by TCs is the highest in SCLCs and the lowest in ACs. IC infiltration is the least pronounced in AC lesions, and IC VISTA and GITR expressions are also considerably lower in these intermediate-grade malignancies. Altogether, these results might aid in designing clinical trials evaluating the efficacy of particular immunotherapeutics directed against VISTA, OX40L, GITR, and TIM3 in these hard-to-treat malignancies.
Data availability
Data were generated by the authors and are available upon reasonable request.
References
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F (2021) Cancer statistics for the year 2020: an overview. Int J Cancer. https://doi.org/10.1002/ijc.33588
Metovic J, Barella M, Bianchi F et al (2021) Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch 478:5–19. https://doi.org/10.1007/s00428-020-03015-z
Nicholson AG, Tsao MS, Beasley MB et al (2022) The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol 17:362–387. https://doi.org/10.1016/j.jtho.2021.11.003
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Travis WD, Brambilla E, Nicholson AG et al (2015) The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10:1243–1260. https://doi.org/10.1097/jto.0000000000000630
Morandi U, Casali C, Rossi G (2006) Bronchial typical carcinoid tumors. Semin Thorac Cardiovasc Surg 18:191–198. https://doi.org/10.1053/j.semtcvs.2006.08.005
Baudin E, Caplin M, Garcia-Carbonero R et al (2021) Lung and thymic carcinoids: ESMO clinical practice guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol 32:439–451. https://doi.org/10.1016/j.annonc.2021.01.003
Pusceddu S, Lo Russo G, Macerelli M et al (2016) Diagnosis and management of typical and atypical lung carcinoids. Crit Rev Oncol Hematol 100:167–176. https://doi.org/10.1016/j.critrevonc.2016.02.009
Randhawa S, Trikalinos N, Patterson GA (2021) Neuroendocrine tumors of the lung. Thorac Surg Clin 31:469–476. https://doi.org/10.1016/j.thorsurg.2021.05.005
Ferrara MG, Stefani A, Simbolo M et al (2021) Large cell neuro-endocrine carcinoma of the lung: current treatment options and potential future opportunities. Front Oncol 11:650293. https://doi.org/10.3389/fonc.2021.650293
Borczuk AC (2020) Pulmonary neuroendocrine tumors. Surg Pathol Clin 13(1):35–55. https://doi.org/10.1016/j.path.2019.10.002
Megyesfalvi Z, Gay CM, Popper H et al (2023) Clinical insights into small cell lung cancer: tumor heterogeneity, diagnosis, therapy, and future directions. CA: A Cancer J Clin 73(6):620–652. https://doi.org/10.3322/caac.21785
Lim SM, Hong MH, Kim HR (2020) Immunotherapy for non-small cell lung cancer: current landscape and future perspectives. immune Netw 20:e10. https://doi.org/10.4110/in.2020.20.e10
Reck M, Remon J, Hellmann MD (2022) First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol 40:586–597. https://doi.org/10.1200/jco.21.01497
Albertelli M, Dotto A, Nista F, Veresani A, Patti L, Gay S, Sciallero S, Boschetti M, Ferone D (2021) Present and future of immunotherapy in neuroendocrine tumors. Rev Endocr Metab Disord 22:615–636. https://doi.org/10.1007/s11154-021-09647-z
Klein O, Kee D, Markman B et al (2020) Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res 26:4454–4459. https://doi.org/10.1158/1078-0432.Ccr-20-0621
Di Molfetta S, Feola T, Fanciulli G, Florio T, Colao A, Faggiano A, Nike G (2022) Immune checkpoint blockade in lung carcinoids with aggressive behaviour: one more arrow in our quiver? J Clin Med. https://doi.org/10.3390/jcm11041019
Berghmans T, Dingemans AM, Hendriks LEL, Cadranel J (2020) Immunotherapy for nonsmall cell lung cancer: a new therapeutic algorithm. Eur Respir J. https://doi.org/10.1183/13993003.01907-2019
Pavan A, Attili I, Pasello G, Guarneri V, Conte PF, Bonanno L (2019) Immunotherapy in small-cell lung cancer: from molecular promises to clinical challenges. J Immunother Cancer 7:205. https://doi.org/10.1186/s40425-019-0690-1
Dantoing E, Piton N, Salaün M, Thiberville L, Guisier F (2021) Anti-PD1/PD-L1 immunotherapy for non-small cell lung cancer with actionable oncogenic driver mutations. Int J Mol Sci. https://doi.org/10.3390/ijms22126288
Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 10:727–742
Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B (2020) CTLA-4: From mechanism to autoimmune therapy. Int Immunopharmacol 80:106221. https://doi.org/10.1016/j.intimp.2020.106221
Rowshanravan B, Halliday N, Sansom DM (2018) CTLA-4: a moving target in immunotherapy. Blood 131:58–67. https://doi.org/10.1182/blood-2017-06-741033
Ferencz B, Megyesfalvi Z, Csende K et al (2023) Comparative expression analysis of immune-related markers in surgically resected lung neuroendocrine neoplasms. Lung Cancer 181:107263. https://doi.org/10.1016/j.lungcan.2023.107263
Huang X, Zhang X, Li E, Zhang G, Wang X, Tang T, Bai X, Liang T (2020) VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol 13:83. https://doi.org/10.1186/s13045-020-00917-y
Mortezaee K, Majidpoor J, Najafi S (2022) VISTA immune regulatory effects in bypassing cancer immunotherapy: updated. Life Sci 310:121083. https://doi.org/10.1016/j.lfs.2022.121083
Tagliamento M, Agostinetto E, Borea R, Brandão M, Poggio F, Addeo A, Lambertini M (2021) VISTA: a promising target for cancer immunotherapy? Immunotargets Ther 10:185–200. https://doi.org/10.2147/itt.S260429
Lu X (2021) OX40 and OX40L interaction in cancer. Curr Med Chem 28:5659–5673. https://doi.org/10.2174/0929867328666201229123151
Redmond WL, Weinberg AD (2007) Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol 27:415–436. https://doi.org/10.1615/critrevimmunol.v27.i5.20
Chan S, Belmar N, Ho S et al (2022) An anti-PD-1-GITR-L bispecific agonist induces GITR clustering-mediated T cell activation for cancer immunotherapy. Nat Cancer 3:337–354. https://doi.org/10.1038/s43018-022-00334-9
Hernandez-Guerrero T, Moreno V (2022) GITR Antibodies in cancer: not ready for prime time. Clin Cancer Res 28:3905–3907. https://doi.org/10.1158/1078-0432.Ccr-22-1489
Solinas C, De Silva P, Bron D, Willard-Gallo K, Sangiolo D (2019) Significance of TIM3 expression in cancer: from biology to the clinic. Semin Oncol 46:372–379. https://doi.org/10.1053/j.seminoncol.2019.08.005
Kandel S, Adhikary P, Li G, Cheng K (2021) The TIM3/Gal9 signaling pathway: an emerging target for cancer immunotherapy. Cancer Lett 510:67–78. https://doi.org/10.1016/j.canlet.2021.04.011
Buzzatti G, Dellepiane C, Del Mastro L (2020) New emerging targets in cancer immunotherapy: the role of GITR. ESMO Open 4:e000738. https://doi.org/10.1136/esmoopen-2020-000738
Davar D, Zappasodi R (2023) Targeting GITR in cancer immunotherapy - there is no perfect knowledge. Oncotarget 14:614–621. https://doi.org/10.18632/oncotarget.28461
Herrera-Camacho I, Anaya-Ruiz M, Perez-Santos M, Millán-Pérez Peña L, Bandala C, Landeta G (2019) Cancer immunotherapy using anti-TIM3/PD-1 bispecific antibody: a patent evaluation of EP3356411A1. Expert Opin Ther Pat 29:587–593. https://doi.org/10.1080/13543776.2019.1637422
Martin AS, Molloy M, Ugolkov A, von Roemeling RW, Noelle RJ, Lewis LD, Johnson M, Radvanyi L, Martell RE (2023) VISTA expression and patient selection for immune-based anticancer therapy. Front Immunol 14:1086102. https://doi.org/10.3389/fimmu.2023.1086102
Mlika M, Zendah I, Braham E, El Mezni F (2015) CD56 antibody: old-fashioned or still trendy in endocrine lung tumors. J Immunoassay Immunochem 36:414–419. https://doi.org/10.1080/15321819.2014.952444
Pelosi G, Rindi G, Travis WD, Papotti M (2014) Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 9:273–284. https://doi.org/10.1097/jto.0000000000000092
Xiao Y, Yu D (2021) Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 221:107753. https://doi.org/10.1016/j.pharmthera.2020.107753
Whiteside TL (2010) Immune responses to malignancies. J Allergy Clin Immunol 125:S272–S283. https://doi.org/10.1016/j.jaci.2009.09.045
Galli F, Aguilera JV, Palermo B, Markovic SN, Nisticò P, Signore A (2020) Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res 39:89. https://doi.org/10.1186/s13046-020-01586-y
Ruiz-Cordero R, Devine WP (2020) Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin 13:17–33. https://doi.org/10.1016/j.path.2019.11.002
Gonzalez H, Hagerling C, Werb Z (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32:1267–1284. https://doi.org/10.1101/gad.314617.118
Bruni D, Angell HK, Galon J (2020) The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer 20:662–680. https://doi.org/10.1038/s41568-020-0285-7
Moonen L, Derks J, Dingemans AM, Speel EJ (2019) Orthopedia homeobox (OTP) in Pulmonary neuroendocrine tumors: the diagnostic value and possible molecular interactions. Cancers (Basel). https://doi.org/10.3390/cancers11101508
Papaxoinis G, Nonaka D, O’Brien C, Sanderson B, Krysiak P, Mansoor W (2017) Prognostic significance of CD44 and Orthopedia homeobox protein (OTP) expression in pulmonary carcinoid tumours. Endocr Pathol 28:60–70. https://doi.org/10.1007/s12022-016-9459-y
Swarts DR, Henfling ME, Van Neste L et al (2013) CD44 and OTP are strong prognostic markers for pulmonary carcinoids. Clin Cancer Res 19:2197–2207. https://doi.org/10.1158/1078-0432.Ccr-12-3078
Swarts DR, Scarpa A, Corbo V et al (2014) MEN1 gene mutation and reduced expression are associated with poor prognosis in pulmonary carcinoids. J Clin Endocrinol Metab 99:E374–E378. https://doi.org/10.1210/jc.2013-2782
Derks JL, Leblay N, Lantuejoul S, Dingemans AC, Speel EM, Fernandez-Cuesta L (2018) New insights into the molecular characteristics of pulmonary carcinoids and large cell neuroendocrine carcinomas, and the impact on their clinical management. J Thorac Oncol 13:752–766. https://doi.org/10.1016/j.jtho.2018.02.002
George J, Walter V, Peifer M et al (2018) Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun 9:1048. https://doi.org/10.1038/s41467-018-03099-x
Rekhtman N, Pietanza MC, Hellmann MD et al (2016) Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res 22:3618–3629. https://doi.org/10.1158/1078-0432.Ccr-15-2946
Rekhtman N (2022) Lung neuroendocrine neoplasms: recent progress and persistent challenges. Mod Pathol 35:36–50. https://doi.org/10.1038/s41379-021-00943-2
Gay CM, Stewart CA, Park EM et al (2021) Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 39:346–60.e7. https://doi.org/10.1016/j.ccell.2020.12.014
Rudin CM, Poirier JT, Byers LA et al (2019) Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 19:289–297. https://doi.org/10.1038/s41568-019-0133-9
Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L (2014) VISTA regulates the development of protective antitumor immunity. Cancer Res 74:1933–1944. https://doi.org/10.1158/0008-5472.Can-13-1506
Hendry S, Salgado R, Gevaert T et al (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: tils in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol 24:311–335. https://doi.org/10.1097/pap.0000000000000161
Zong L, Mo S, Sun Z, Lu Z, Yu S, Chen J, Xiang Y (2022) Analysis of the immune checkpoint V-domain Ig-containing suppressor of T-cell activation (VISTA) in endometrial cancer. Mod Pathol 35:266–273. https://doi.org/10.1038/s41379-021-00901-y
Muller S, Victoria Lai W, Adusumilli PS et al (2020) V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod Pathol 33:303–311. https://doi.org/10.1038/s41379-019-0364-z
Terenziani R, Zoppi S, Fumarola C, Alfieri R, Bonelli M (2021) Immunotherapeutic approaches in malignant pleural mesothelioma. Cancers (Basel). https://doi.org/10.3390/cancers13112793
Saleh R, Taha RZ, Toor SM et al (2020) Expression of immune checkpoints and T cell exhaustion markers in early and advanced stages of colorectal cancer. Cancer Immunol Immunother 69:1989–1999. https://doi.org/10.1007/s00262-020-02593-w
Yuan L, Tatineni J, Mahoney KM, Freeman GJ (2021) VISTA: a mediator of quiescence and a promising target in cancer immunotherapy. Trends Immunol 42:209–227. https://doi.org/10.1016/j.it.2020.12.008
Röcken C (2023) Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol 149:467–481. https://doi.org/10.1007/s00432-022-04408-0
Hung YP (2019) Neuroendocrine tumors of the lung: updates and diagnostic pitfalls. Surg Pathol Clin 12:1055–1071. https://doi.org/10.1016/j.path.2019.08.012
Wang Y, Zhang H, Liu C et al (2022) Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts. J Hematol Oncol 15:111. https://doi.org/10.1186/s13045-022-01325-0
Rittig SM, Lutz MS, Clar KL et al (2022) Controversial role of the immune checkpoint OX40L expression on platelets in breast cancer progression. Front Oncol 12:917834. https://doi.org/10.3389/fonc.2022.917834
Fu Y, Lin Q, Zhang Z, Zhang L (2020) Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm Sin B 10:414–433. https://doi.org/10.1016/j.apsb.2019.08.010
Porciuncula A, Morgado M, Gupta R, Syrigos K, Meehan R, Zacharek SJ, Frederick JP, Schalper KA (2021) Spatial Mapping and immunomodulatory role of the OX40/OX40L Pathway in human non-small cell lung cancer. Clin Cancer Res 27:6174–6183. https://doi.org/10.1158/1078-0432.Ccr-21-0987
Chen P, Wang H, Zhao L et al (2021) Immune checkpoints OX40 and OX40L in small-cell lung cancer: predict prognosis and modulate immune microenvironment. Front Oncol 11:713853. https://doi.org/10.3389/fonc.2021.713853
Chen X, Ma H, Mo S, Zhang Y, Lu Z, Yu S, Chen J (2022) Analysis of the OX40/OX40L immunoregulatory axis combined with alternative immune checkpoint molecules in pancreatic ductal adenocarcinoma. Front Immunol 13:942154. https://doi.org/10.3389/fimmu.2022.942154
Nocentini G, Riccardi C (2009) GITR: a modulator of immune response and inflammation. Adv Exp Med Biol 647:156–173. https://doi.org/10.1007/978-0-387-89520-8_11
Zappasodi R, Sirard C, Li Y et al (2019) Rational design of anti-GITR-based combination immunotherapy. Nat Med 25:759–766. https://doi.org/10.1038/s41591-019-0420-8
Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T (2022) Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol 19:37–50. https://doi.org/10.1038/s41571-021-00552-7
Ronchetti S, Nocentini G, Petrillo MG, Bianchini R, Sportoletti P, Bastianelli A, Ayroldi EM, Riccardi C (2011) Glucocorticoid-Induced TNFR family related gene (GITR) enhances dendritic cell activity. Immunol Lett 135:24–33. https://doi.org/10.1016/j.imlet.2010.09.008
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 11:39. https://doi.org/10.1186/s13045-018-0582-8
Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C (2007) GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol 37:1165–1169. https://doi.org/10.1002/eji.200636933
Riccardi C, Ronchetti S, Nocentini G (2018) Glucocorticoid-induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin Ther Targets 22:783–797. https://doi.org/10.1080/14728222.2018.1512588
Zhao L, Cheng S, Fan L, Zhang B, Xu S (2021) TIM-3: an update on immunotherapy. Int Immunopharmacol 99:107933. https://doi.org/10.1016/j.intimp.2021.107933
Wolf Y, Anderson AC, Kuchroo VK (2020) TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol 20:173–185. https://doi.org/10.1038/s41577-019-0224-6
Grillo F, Bruzzone M, Pigozzi S, Prosapio S, Migliora P, Fiocca R, Mastracci L (2017) Immunohistochemistry on old archival paraffin blocks: is there an expiry date? J Clin Pathol 70:988–993. https://doi.org/10.1136/jclinpath-2017-204387
Kokkat TJ, Patel MS, McGarvey D, LiVolsi VA, Baloch ZW (2013) Archived formalin-fixed paraffin-embedded (FFPE) blocks: a valuable underexploited resource for extraction of DNA, RNA, and protein. Biopreserv Biobank 11:101–106. https://doi.org/10.1089/bio.2012.0052
Acknowledgements
We would like to thank Katalin Parraghné Derecskei and Petra Rembeczki for their excellent technical assistance.
Funding
Open access funding provided by Medical University of Vienna. BD, ZM, JB, and BF were supported by funding from the Hungarian National Research, Development, and Innovation Office (KH130356 to BD; 2020‐1.1.6‐JÖVŐ, FK‐143751 and FK-147045 to BD and ZM; TKP2021‐EGA‐33 to BD, ZM, JB, and BF). BD and JB acknowledge funding from the Hungarian Academy of Sciences (PC2022-II-19/1/2022). BD was also supported by the Austrian Science Fund (FWF I3522, FWF I3977, and I4677) and the “BIOSMALL” EU HORIZON-MSCA-2022-SE-01 project. ZM was supported by the New National Excellence Program of the Ministry for Innovation and Technology of Hungary (UNKP‐20‐3, UNKP‐21‐3 and UNKP-23–5), and by the Bolyai Research Scholarship of the Hungarian Academy of Sciences. ZM is also the recipient of an International Association for the Study of Lung Cancer/International Lung Cancer Foundation Young Investigator Grant (2022). BF is a recipient of the Semmelweis 250 + Excellence PhD Scholarship (EFOP-3.6.3-VEKOP-16–2017-00009) of the Semmelweis University. KS was supported by the Austrian Science Fund (FWF No. T 1062-B33) and the City of Vienna Fund for Innovative Interdisciplinary Cancer Research.
Author information
Authors and Affiliations
Contributions
(I) BF, KT, KC, JF, JB, ZM, and BD contributed to study conception and design; (II) all authors were involved in administrative support; (III) FR-V, KS, JB, and BD contributed to provision of study materials; (IV) BF, KT, JF, AL, OP, JB, and ZM were involved in collection and assembly of data; (V) BF, KC, JF, AL, OP, JB, and ZM contributed to data analysis and interpretation; (VI) all authors were involved in manuscript writing; and (VII) all authors contributed to final approval of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The study was conducted in accordance with the guidelines of the Helsinki Declaration of the World Medical Association and with the approval of the national-level Ethics Committee of each participating country. Patient identifiers were removed after clinical data collection to ensure patient pseudonymity. The requirement for written informed consent was waived due to the study’s retrospective nature.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Balázs Döme and Zsolt Megyesfalvi share the senior authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferencz, B., Török, K., Pipek, O. et al. Expression patterns of novel immunotherapy targets in intermediate- and high-grade lung neuroendocrine neoplasms. Cancer Immunol Immunother 73, 114 (2024). https://doi.org/10.1007/s00262-024-03704-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-024-03704-7