Abstract
Immune checkpoint inhibition (ICI) can induce durable responses in patients with advanced malignancies. Three cases of hematological neoplasia following ICI for solid tumors have been reported to date. We present five patients treated at our tertiary referral center between 2017 and 2021 who developed chronic myeloid leukemia (two patients), acute myeloid leukemia, myelodysplastic syndrome and chronic eosinophilic leukemia during or after anti-PD-1-based treatment. Molecular analyses were performed on pre-ICI samples to identify baseline variants in myeloid genes. We hypothesize that PD-1 blockade might accelerate progression to overt myeloid malignancies and discuss potential underlying mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibition (ICI) targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed cell death [ligand] 1 (PD-[L]1) can induce durable responses in some patients with advanced malignancies. A well-established downside of ICI is its diverse spectrum of immune-related adverse events (irAEs). If ICI therapy reduces or increases second primary malignancy incidence, has been a matter of debate [1, 2]. Here, we present five patients treated at our academic center between 2017 and 2021 who developed clinically manifest myeloid neoplasia during or after ICI treatment for solid tumors. Two patients developed chronic myeloid leukemia (CML), one acute myeloid leukemia (AML), one myelodysplastic syndrome (MDS) and one chronic eosinophilic leukemia (CEL). For three patients we performed molecular analyses on baseline material, to identify baseline variants in myeloid genes. Lastly, we discuss these findings in the light of possible links between ICI and the subsequent manifestation of myeloid neoplasia, along with alternative explanations.
Case series
Clinical characteristics and complete blood counts (CBC) are displayed in Table 1 and Fig. 1, respectively.
Patient 1 is a man in mid-60s with a history of anti-CCP-negative rheumatoid arthritis and ulcerative colitis in remission without treatment. He developed stage IV melanoma which progressed after dacarbazine long ago and was treated with one cycle of ipilimumab, followed by pembrolizumab for 11 months [3], resulting in good partial response. Two years after pembrolizumab discontinuation, leukocytosis (67.4 × 109/L) was found (hemoglobin level [Hb] 9.4 mmol/L, platelet count [PLT] 255 × 109/L). Bone marrow biopsy showed marked granulocytic proliferation with < 1% blasts. Fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR) detected the BCR-ABL1 fusion gene, without additional cytogenetic aberrations. CML (low Sokal score) was diagnosed and, eventually, dasatinib 100 mg q.d. resulted in optimal response. During three-year follow-up, optimal response to dasatinib persisted, without melanoma progression. FISH performed on lymphocytes (since granulocytes were scarce) from a paraffin-embedded lymph node biopsy obtained before pembrolizumab initiation did not reveal BCR-ABL1.
Patient 2, a man in mid-50s, was diagnosed with stage IV BRAF mutant melanoma with brain and liver metastases. After resection and radiotherapy of the brain metastasis, pembrolizumab was started. Upon initiation of pembrolizumab, leukocytes and platelets were 11.6 × 109/L and 446 × 109/L, respectively. After four months, patient developed leukocytosis (44.8 × 109/L) with normal differentiation (Hb 7.9 mmol/L, PLT 444 × 109/L). With scans showing complete response of the melanoma metastases, pembrolizumab was stopped after six cycles. FISH and RT-PCR for BCR-ABL1 were positive, without additional cytogenetic abnormalities. The bone marrow was hypercellular with marked granulocytic proliferation, decreased erythropoiesis, small megakaryocytes, and < 5% blasts, supporting the diagnosis of CML (low Sokal score). Although the CML remained in durable remission under nilotinib treatment, patient died one year later from progressive melanoma with leptomeningeal metastases. No BCR-ABL1 fusion could be detected through FISH in granulocytes from a paraffin-embedded brain biopsy obtained pre-ICI.
Patient 3 is a female in mid-70s known with non-small cell lung cancer (NSCLC) for 12 years. After initial radical resection, followed by stereotactic radiotherapy (60 Gy) on a solitary lesion, concurrent chemoradiotherapy (cisplatin/etoposide with 30 × 2 Gy) and durvalumab for a stage IIIB relapse were initiated. Six months later, she underwent a lobectomy with adjuvant chemotherapy (carboplatin/pemetrexed) for clonally unrelated second NSCLC. After three cycles, chemotherapy was stopped for intolerance. Anemia (Hb 5.7–7.3 mmol/L) and thrombocytopenia (PLT ± 100 × 109/L) incompletely recovered after chemotherapy cessation. Because of rapid disease progression, nivolumab monotherapy was initiated, with known anemia, thrombocytopenia and leukocytosis upon cycle 1. Leukocyte differentiation revealed mainly segmented neutrophils, 11% monocytes, 1% myelocytes and promyelocytes, but no blasts. Two weeks later, before the second nivolumab cycle, leukocytosis (27.3 × 109/L) was found with 39% monocytes, and 39% segmented neutrophils and 6% blasts containing Auer rods. Blood immunophenotyping revealed 10% myeloid blasts. AML or MDS with excess blasts (MDS-EB)-2 was suspected, for which the patient wished neither diagnostics nor treatment. After several weeks she succumbed. Molecular analysis by next-generation sequencing (NGS) of a preserved cell pellet from the time of AML onset revealed pathogenic variants in NRAS (VAF 9%) and RUNX1 (VAF 6.4%), and a variant of unknown significance (VUS) in CEBPA (VAF 6%). NGS analysis of PBMCs collected, while 1.5 months on durvalumab treatment demonstrated 100% wild-type allele frequencies of all three genes.
Patient 4 is a man in mid-60s with metastatic melanoma for which combined ipilimumab and nivolumab were started. At immunotherapy initiation, CBC was unremarkable (leukocytes 6.0 × 109/L, Hb 8.8 mmol/L, PLT 156 × 109/L). Stable disease was achieved after 4 cycles, while over the course of one year anemia slowly developed (Hb 6.1 mmol/L upon nivolumab cycle 11). Bone marrow investigation demonstrated trilinear dysplasia and 9% CD34+ blasts. MDS-EB1 was concluded with a minimal R-ISS score of 4 (intermediate risk). Given ineligibility for allogeneic stem cell transplantation, cytogenetic evaluation was waived. Instead, best supportive care including subcutaneous darbepoetin 150 µg/week was initiated and seven weeks later increased to 300 µg/week for persisting anemia (Hb ≤ 5.5 mmol/L).
Patient 5, a man in his 80s, had a history of polymyalgia rheumatica, for which he used 10 mg prednisone daily, and metastatic clear-cell renal cell carcinoma (without pulmonary metastases) for which combined ipilimumab and nivolumab were started. Ten months afterward, he presented at the emergency department with progressive dyspnea. Laboratory analysis revealed severe hypereosinophilia (35.6 × 109/L), with Hb 9.4 mmol/L, PLT 147 × 109/L, 9.18 × 109/L segmented neutrophils, 0.54 × 109/L basophils, 1.62 × 109/L monocytes and 6.48 × 109/L lymphocytes. Since eosinophilia is frequently seen in the context of irAEs, prednisone 2 mg/kg was pragmatically started. Nevertheless, eosinophils rose to 104 × 109/L over several days and first decreased after the start of hydroxycarbamide. Blood and bone marrow immunophenotyping revealed 0.65% and 0.40% monoclonal IgM lambda-positive B cells, respectively, suspect for hairy cell leukemia variant. However, related lymphocytic hypereosinophilic syndrome (HES) was deemed unlikely given absence of a T cell clone and low serum interleukin-5 and G-CSF. The bone marrow was hypercellular (85% cellularity) due to marked eosinophilic proliferation without blast increase. T lymphocytes were normal. SRSF2 (VAF 40%) and NPM1 mutations (VAF 35%) were found and imatinib, later replaced by dasatinib, was added for suspected CEL not otherwise specified. Despite further decrease following tyrosine kinase inhibitors (TKIs), eosinophils did not reach the normal range. After initiation of anticoagulants for newly developed pulmonary embolisms, hydroxycarbamide was discontinued. With alleged unavoidable short-term progression to AML [4], patient was referred to a hospice. Remarkably, two weeks after hospice admission he recovered clinically. Seven weeks later, while on steroids, leukocytosis had improved (14 × 109/L). Shortly after, patient died of complicated COVID-19 infection.
Discussion
Between 2017 and 2021, we encountered five cases of myeloid neoplasia developing during or shortly after ICI treatment (PD-[L]1 inhibitors with or without CTLA-4 inhibitors). A pharmacovigilance study previously detected no statistically significant disproportionality signal for AML or CML and ICI [5].
Alternative mechanisms should be considered. Etoposide exposure 19 months before AML in the third patient suggests a chemotherapy-induced etiology [6]. Nevertheless, in this case the acute leukemic blast crisis, remarkably, developed immediately after the first cycle of nivolumab. Regarding patient 1, CML has not been associated with prior chemotherapy exposure and secondary cancer incidence was shown not to be increased after dacarbazine in the context of the ABVD regimen without irradiation for Hodgkin’s lymphoma [7]. Although HES has been described as an immune-related phenomenon, peak eosinophil counts in previous cases were significantly lower than in patient 5 [8]. Moreover, previous HES patients demanding pharmacological management generally showed rapid responses to steroids [8], whereas patient 5 required hydroxycarbamide and TKIs beyond steroids with the first decrease in eosinophils after 8 days. Together with proven driver mutations [4], we therefore considered CEL-NOS more likely than a severe irAE.
We found no pre-ICI molecular aberrations in patients 1–3. This could indicate that at baseline pathogenic mutations were absent in all three patients and malignant transformation had yet to occur upon durvalumab initiation in patient 3. However, for the lack of preferential baseline biospecimens, low cell yield and the absence of bone marrow samples, these analyses cannot rule out myeloid somatic variants or the presence of subclinical malignant clones at baseline. Importantly, results do not preclude the possibility that ICI contributes to shorter time-to-manifestation of myeloid neoplasia.
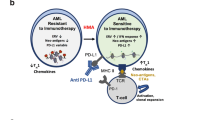
Both preclinical [9] and clinical [10, 11] evidence support that PD-1 blockade can lead to secondary lymphoproliferative disease. Indeed, one hypothesis suggests that mutant T cell clones are provided with an additional proliferation advantage following lymphoid PD-1 blockade [10]. However, regarding myeloid lineage, myeloid-specific PD-1 ablation in a murine melanoma model enhanced effector T response and led to a larger decrease in tumor growth than T-cell-specific PD-1 ablation [12]. Yet, ICI mechanisms of action have proven complex and many theories explaining non-response or hyperprogression have been developed, including ‘tumor-intrinsic PD-1 signaling.’ Kim et al. [13] reported a case of acute myelomonocytic leukemia (AMML) after three cycles of pembrolizumab for NSCLC. The authors hypothesized hyperprogression of subclinical AMML as a potential explanation. In such theory, a myeloid clone with acquired driver mutation(s) could obtain an extra proliferation advantage from functional myeloid PD-1 knockout after ICI. Analogically, in CML the BCR-ABL1 fusion is the only mandatory molecular criterion for CML. Additional events, however, are probably necessary for progression to overt CML, since some healthy individuals carry BCR-ABL1 [14].
PDCD1 transcription has been demonstrated in over 30 cancers, including hematological malignancies [15]. Surface PD-1 expression could be confirmed in subsets of cells in 40 different cancer cell lines by flow cytometry [15]. Specifically, abberant PD-1 expression has been demonstrated in 8–26% of CD34+ blasts in MDS, CMML and AML [16]. Although the latter study found no difference in AML/MDS overall survival depending on PD-1 expression in blasts [16], another study in AML reported that higher PD-1 expression on leukemic blasts was associated with longer disease-free survival [17]. In MDS, an approximately fourfold larger fraction of CD34+ blasts was PD-1+ compared to healthy control bone marrow [18]. This upregulation of the PD-1/PD-L1 axis was shown to contribute to premature death of hematopoietic progenitors in MDS, while hematopoiesis could be recovered by PD-1/PD-L1 blockade in aged S100A9 transgenic mice (which phenotypically resemble MDS) and human MDS bone marrow mononuclear cells [18]. Although such restoration of HSC survival may appear favorable, as it presents possibilities for MDS treatment, it could also contribute to clonal selection of malignantly transformed progenitors.
In T lymphocytes, PD-1/PD-L1/2 ligation results in SHP-2 binding, followed by CD28 and T cell receptor dephosphorylation [19]. In contrast, non-canonical PD-1 signaling has been demonstrated in cancer cells, resulting in both protumor and tumor-suppressing effects [19]. Protumor effects of intrinsic PD-1 have been shown through multiple mechanisms including enhanced mTOR signaling and NFκB activation in some tumors and may even act indepently of PD-1/PD-L1 ligation [20,21,22,23]. On the contrary, in vitro and in vivo PDCD1 knockdown models, as well as immunodeficient mice inoculated with lung cancer cell lines and treated with anti-PD-1, showed increased colony expansion and tumor growth [15, 24], mediated through upregulated AKT and ERK signaling [15]. Along these lines, p53 has been shown to transcriptionally regulate PD-1 expression in cancer cells and induced cancer cell intrinsic PD-1 expression could inhibit tumor growth in mice [25]. These experimental data support the hypothesis that anti-PD-1 may functionally knock-out tumor-intrisic PD-1 and lead to accelarated clonal expansion of myeloid malignant cells.
Besides tumor intrinsic PD-1 signaling, the inflammatory milieu may also mediate clonal expansion through ICI-induced cytokines or chemokines that sort effects in particular myeloid subsets. In acute inflammation, IFN-γ and GM-CSF increase myeloid differentiation bias and chronic inflammation may pose selective pressure to premalignant clones [26]. Elevated IL-16 and CCL2 after ICI treatment have been associated with increased eosinophil accumulation [27]. However, the complexity of cytokine responses following ICI, in rare cases escalating to cytokine release syndrome [28], prevents definite statements on which effects cytokines have on myeloid clonal selection.
In conclusion, PD-1 blockade might accelerate progression to overt myeloid malignancies, for example in one of the above-hypothesized ways. Although we could not elucidate the exact mechanism, the increasing number of patients treated with ICIs and incomplete understanding of ICI mechanisms warrants attention for the above cases. Prospective studies into this particular matter will not be feasible given relatively low incidence and required follow-up. Instead, pharmacovigilance studies, preclinical models and studies examining immune response after ICI can help reveal if a particular mechanism underlies our clinical observations.
Abbreviations
- Hb:
-
Hemoglobin level
- ICI:
-
Immune checkpoint inhibition
- irAE:
-
Immune-related adverse event
- PLT:
-
Platelet count
- TKI:
-
Tyrosine kinase inhibitor
- VAF:
-
Variant allele frequency
References
Heudel P, Chabaud S, Perol D, Ray-Coquard I, Blay JY (2020) Reduced risk of second primary cancer in patients treated with immune checkpoint inhibitors for a first cancer. Ann Oncol 31(12):1773–1775. https://doi.org/10.1016/j.annonc.2020.09.001
Suijkerbuijk KPM, May AM, van Eijs MJM (2021) Checkpoint inhibition: Protecting against or predisposing for second primary tumors? Ann Oncol 32(7):935. https://doi.org/10.1016/j.annonc.2021.03.202
Meerveld-Eggink A, Rozeman EA, Lalezari F, van Thienen JV, Haanen JBAG, Blank CU (2017) Short-term CTLA-4 blockade directly followed by PD-1 blockade in advanced melanoma patients: a single-center experience. Ann Oncol 28(4):862–7. https://doi.org/10.1093/annonc/mdw692
Hofmans M, Delie A, Vandepoele K et al (2018) A case of chronic eosinophilic leukemia with secondary transformation to acute myeloid leukemia. Leuk Res Rep 9:45–47. https://doi.org/10.1016/j.lrr.2018.04.001
Ye X, Hu F, Zhai Y et al (2020) Hematological toxicities in immune checkpoint inhibitors: a pharmacovigilance study from 2014 to 2019. Hematol Oncol 38(4):565–575. https://doi.org/10.1002/hon.2743
Pui CH, Relling MV (2000) Topoisomerase II inhibitor-related acute myeloid leukemia. Br J Haematol 109(1):13–23. https://doi.org/10.1046/j.1365-2141.2000.01843.x
Swerdlow AJ, Higgins CD, Smith P et al (2011) Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol 29(31):4096–4104. https://doi.org/10.1200/JCO.2011.34.8268
Scanvion Q, Béné J, Gautier S et al (2020) Moderate-to-severe eosinophilia induced by treatment with immune checkpoint inhibitors: 37 cases from a national reference center for hypereosinophilic syndromes and the French pharmacovigilance database. Oncoimmunology 9(1):e1722022. https://doi.org/10.1080/2162402X.2020.1722022
Wartewig T, Kurgyis Z, Keppler S et al (2017) PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 552(7683):121–125. https://doi.org/10.1038/nature24649
Anand K, Ensor J, Pingali SR et al (2020) T-cell lymphoma secondary to checkpoint inhibitor therapy. J Immunother Cancer 8(1):e000104. https://doi.org/10.1136/jitc-2019-000104
Marumo Y, Kusumoto S, Masaki A et al (2021) Newly diagnosed follicular lymphoma during pembrolizumab treatment for lung cancer. Int J Hematol 114(2):280–285. https://doi.org/10.1007/s12185-021-03135-5
Strauss L, Mahmoud MAA, Weaver JD et al (2020) Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 5(43):eaay1863. https://doi.org/10.1126/sciimmunol.aay1863
Kim H-B, Park S-G, Hong R, Kang S-H, Na YS (2020) Acute myelomonocytic leukemia during pembrolizumab treatment for non-small cell lung cancer: a case report. World J Clin Cases 8(13):2833–2840
Kuan JW, Su AT, Leong CF, Osato M, Sashida G (2020) Systematic review of normal subjects harbouring BCR-ABL1 fusion gene. Acta Haematol 143(2):96–111. https://doi.org/10.1159/000501146
Wang X, Yang X, Zhang C et al (2020) Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc Natl Acad Sci USA 117(12):6640–6650. https://doi.org/10.1073/pnas.1921445117
Yang H, Bueso-Ramos C, DiNardo C et al (2014) Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 28(6):1280–1288. https://doi.org/10.1038/leu.2013.355
Schmohl JU, Nuebling T, Wild J et al (2016) Expression of RANK-L and in part of PD-1 on blasts in patients with acute myeloid leukemia correlates with prognosis. Eur J Haematol 97(6):517–527. https://doi.org/10.1111/ejh.12762
Cheng P, Eksioglu EA, Chen X et al (2019) S100A9-induced overexpression of PD-1/PD-L1 contributes to ineffective hematopoiesis in myelodysplastic syndromes. Leukemia 33(8):2034–2046. https://doi.org/10.1038/s41375-019-0397-9
Zha H, Jiang Y, Wang X et al (2021) Non-canonical PD-1 signaling in cancer and its potential implications in clinic. J Immunother Cancer 9(2):e001230. https://doi.org/10.1136/jitc-2020-001230
Pu N, Gao S, Yin H et al (2019) Cell-intrinsic PD-1 promotes proliferation in pancreatic cancer by targeting CYR61/CTGF via the hippo pathway. Cancer Lett 460:42–53. https://doi.org/10.1016/j.canlet.2019.06.013
Li H, Li X, Liu S et al (2017) Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology 66(6):1920–1933. https://doi.org/10.1002/hep.29360
Kleffel S, Posch C, Barthel SR et al (2015) Melanoma cell-Intrinsic PD-1 receptor functions promote tumor growth. Cell 162(6):1242–1256. https://doi.org/10.1016/j.cell.2015.08.052
Mirzaei R, Gordon A, Zemp FJ et al (2021) PD-1 independent of PD-L1 ligation promotes glioblastoma growth through the NFκB pathway. Sci Adv 7(45):eabh2148. https://doi.org/10.1126/sciadv.abh2148
Du S, McCall N, Park K et al (2018) Blockade of tumor-expressed PD-1 promotes lung cancer growth. Oncoimmunology 7(4):e1408747. https://doi.org/10.1080/2162402X.2017.1408747
Cao Z, Kon N, Liu Y et al (2021) An unexpected role for p53 in regulating cancer cell-intrinsic PD-1 by acetylation. Sci Adv 7(14):eabf4148. https://doi.org/10.1126/sciadv.abf4148
Caiado F, Pietras EM, Manz MG (2021) Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J Exp Med 218(7):e20201541. https://doi.org/10.1084/jem.20201541
Simon SCS, Hu X, Panten J et al (2020) Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology 9(1):1727116. https://doi.org/10.1080/2162402X.2020.1727116
Tay SH, Toh MMX, Thian YL et al (2022) Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: a case series of 25 patients and review of the literature. Front Immunol 13:807050. https://doi.org/10.3389/fimmu.2022.807050
Acknowledgements
We thank the Central Diagnostic Laboratory and Department of pathology for generously conducting and providing baseline molecular and cytogenetic analyses.
Funding
This research did not receive any specific funding.
Author information
Authors and Affiliations
Contributions
ME, KS, RM, SN and LW conceptualized the study. RL, LC helped in molecular and cytogenetic analyses. KS supervised ths study. ME wrote the original draft. ME, LW, RM, RL, LC, AL, BS, AK, SN, KS reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KS: advisory relationships with Bristol-Myers Squibb, Novartis, MSD, Pierre Fabre and Abbvie and honoraria from Novartis and Roche, all outside the submitted work and paid to institution. All other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Eijs, M.J.M., van der Wagen, L.E., Mous, R. et al. Hematologic malignancies following immune checkpoint inhibition for solid tumors. Cancer Immunol Immunother 72, 249–255 (2023). https://doi.org/10.1007/s00262-022-03230-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03230-4