Abstract
Background
The optimal treatment duration of ICIs for patients with advanced NSCLC remains uncertain. In phase 3 clinical trials, treatment continued for 2 years or until disease progression with similar long-term survival rates. Real-life data are missing.
Patients and methods
This academic multicentric retrospective study aims at analyzing the characteristics of patients who discontinued treatment after at least 18 months of ICI monotherapy, in the setting of controlled disease.
Results
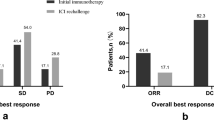
Of the 1127 patients treated with immunotherapy in the given period in six centers, 107 patients had their tumor controlled after at least 18 months of treatment and 54 (50%) of them had discontinued ICI. The median duration of treatment was 26 months. Treatment was stopped due to prescriber choice or toxicity in 46% and 22% of cases, respectively. After a median follow-up of 21 months from ICI discontinuation (95% CI 15.0–26.1 months), 18 (33%) patients experienced tumor progression after a median time of 10.0 months (range 2–33). From discontinuation, 12-month overall survival (OS) and progression-free survival (PFS) were 90% (95% CI 77.7–95.7) and 71% (95% CI 56.8–81.5), respectively; 24-month OS and PFS were 84% (95% CI 68.7–92.2) and 63% (95% CI 46.1–76.2), respectively. Duration of disease control after ICI discontinuation was correlated with tumor response at treatment discontinuation: PFS rate at 12 months was 76% after complete response (CR n = 11) or partial response (PR n = 37) and 22% after only stable disease (SD n = 6) as best response, p-value = 0.0002. PFS rate at 12 months was 80% for CR and/or complete metabolic response with 18F-FDG PET/CT (CMR) and 65% for others. Fourteen patients out of the 18 relapse patients received a subsequent treatment: seven with ICI rechallenge (best response 14% PR and 86% SD) and five with localized therapy with 60% CR.
Conclusions
This real-life study provides new insight into long-term outcomes of patients with advanced NSCLC treated with ICI for at least 18 months before treatment discontinuation in the absence of PD. Tumor response and CMR with FDG PET just before therapy discontinuation may be a predictive factor of prolonged disease control upon discontinuation. These results call for caution in discontinuing treatment in patients with stable disease as the best response.





Similar content being viewed by others
Data availability
All data and materials reported can be requested at the corresponding author.
Abbreviations
- CMR:
-
Complete metabolic response
- CR:
-
Complete response
- ICI:
-
Immune checkpoint inhibitor(s)
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PMR:
-
Partial metabolic response
- PR:
-
Partial response
- SD:
-
Stable disease
- SMD:
-
Stable metabolic disease
References
Groot PM, de Wu CC, Carter BW et al (2018) The epidemiology of lung cancer. Transl Lung Cancer Res 7(3):220–233
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics. CA Cancer J Clin 71(1):7–33
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced non-squamous non-small cell lung cancer. N Engl J Med 373(17):1627–1639
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. The Lancet 393(10183):1819–1830
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301
Gettinger S, Horn L, Jackman D et al (2018) Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol 36(17):1675–1684
Garon EB, Hellmann MD, Rizvi NA et al (2019) Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 37(28):2518–2527
Leighl NB, Hellmann MD, Hui R et al (2019) Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med 4:347–357
Brahmer JR, Rodriguez-Abreu D, Robinson AG et al (2020) KEYNOTE-024 5-year OS update: first-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥ 50%. Annals of Oncol 31(Suppl_4):S1142–S1215
Herbst RS, Garon EB, Kim D-W et al (2020) Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the Keynote-010 study. J Clin Oncol 38(14):1580–1590
Waterhouse DM, Garon EB, Chandler J et al (2020) Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non-small-cell lung cancer: checkmate 153. J Clin Oncol 38(33):3863–3873
Nadal E, Massuti B, Dómine M et al (2019) Immunotherapy with checkpoint inhibitors in non-small cell lung cancer: insights from long-term survivors. Cancer Immunol Immunother 68(3):341–352
Yilmaz M, Guven MS (2020) Durable response after discontinuation of nivolumab therapy in the absence of disease progression or toxicity with two advanced NSCLC patients. J Oncol Pharm Pract 26(3):761–767
Kimura H, Araya T, Yoneda T et al (2019) Long-lasting responses after discontinuation of nivolumab treatment for reasons other than tumor progression in patients with previously treated, advanced non-small cell lung cancer. Cancer Commun (Lond) 39(1):1–5
Haratani K, Hayashi H, Chiba Y et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378
Ricciuti B, Genova C, De Giglio A et al (2019) Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 145(2):479–485
McCoach CE, Blumenthal GM, Zhang L et al (2017) Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann Oncol 28:2707–2714
Tan AC, Emmett L, Lo S et al (2018) FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol 29(10):2115–2120
Kong BY, Menzies AM, Saunders CA et al (2016) Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res 29:572–577
Brahmer JR, Drake CG, Wollner I et al (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28(19):3167–3175
Centanni M, Moes DJAR, Trocóniz IF et al (2019) Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 58(7):835–857
Martins F, Sofiya L, Sykiotis GP et al (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16(9):563–580
Bantia S, Choradia N (2018) Treatment duration with immune-based therapies in Cancer: an enigma. J Immunother Cancer 6(1):143
Simonaggio A, Michot JM, Voisin AL et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 5:1310–1317
Niki M, Nakaya A, Kurata T et al (2018) Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget 9(64):32298–32304
Giaj Levra M, Cotté FE, Corre R et al (2020) Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer 140:99–106
Gobbini E, Toffart AT, Pérol M et al (2020) Immune checkpoint inhibitors rechallenge efficacy in non–small-cell lung cancer patients. Clin Lung Cancer 21(5):e497–e510
Funding
This academic study did not receive any financial support.
Author information
Authors and Affiliations
Contributions
GB and MAM initiated the study concept, coordinated the entire study, and wrote the manuscript. GB, MAM, NG, HD, MGL, EGL, FG, MC, and CD collected and helped interpret clinical data. All authors approved the final version of the article, including the authorship list.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in accordance with the ethical standards of the institutional research committee and the latest Declaration of Helsinki. Study ethics approval was obtained on December 9, 2019 (Observational Research Protocol Review Committee), of the Institut Curie, IRB 2215984.
Consent to participate
An information letter was given to each living patient providing him the opportunity to refuse study participation. All individuals agreed to the use of the obtained data for relevant publications.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bilger, G., Girard, N., Doubre, H. et al. Discontinuation of immune checkpoint inhibitor (ICI) above 18 months of treatment in real-life patients with advanced non-small cell lung cancer (NSCLC): INTEPI, a multicentric retrospective study. Cancer Immunol Immunother 71, 1719–1731 (2022). https://doi.org/10.1007/s00262-021-03114-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03114-z
Keywords
Profiles
- Geoffroy Bilger View author profile