Abstract
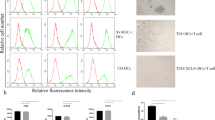
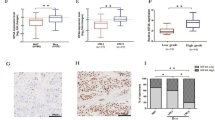
A subset of bladder patients does not respond to BCG treatment effectively and the underlying reason behind this observation is currently unclear. CD4+ T cells are composed of various subsets that each expresses a distinctive set of cytokines and can potently shift the immune response toward various directions. In this study, we examined the CD4+ T-cell cytokine response in bladder cancer patients toward BCG stimulation. We found that bladder cancer patients presented a variety of responses toward BCG, with no uniform characteristics. Those patients with high IFN-γ and IL-21 expression in CD4+ T cells presented significantly better prognosis than patients with low cytokine secretion in CD4+ T cells. Tumor-infiltrating CD4+ T cells were significantly less potent in expressing IFN-γ, IL-4, and IL-17, and more potent in expressing IL-10 than circulating CD4+ T cells. In addition, we found no difference in CD80, CD86, or MHC II expression by macrophages from patients with different IFN-γ and IL-21 levels. However, the secretion of IL-12, a Th1-skewing cytokine, was released at significantly higher level by macrophages from patients with high IFN-γ or high IL-21 secretion. We also identified that modulating monocytes/macrophages by GM-CSF-mediated polarization resulted in significantly elevated expression of IFN-γ and IL-21 from CD4+ T cells. Overall, these results suggested that the specific types of responses mounted by CD4+ T cells were critical to the final outcome of bladder cancer patients and can be influenced by monocyte/macrophage polarization.






Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30. https://doi.org/10.3322/caac.21166
Ingersoll MA, Albert ML (2013) From infection to immunotherapy: host immune responses to bacteria at the bladder mucosa. Mucosal Immunol 6:1041. https://doi.org/10.1038/mi.2013.72
Prescott S, James K, Hargreave TB et al (1992) Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol 147:1636–1642
Babjuk M, Oosterlinck W, Sylvester R et al (2011) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59:997–1008. https://doi.org/10.1016/j.eururo.2011.03.017
Alexandroff AB, Nicholson S, Patel PM, Jackson AM (2010) Recent advances in Bacillus Calmette-Guerin immunotherapy in bladder cancer. Immunotherapy 2:551–560. https://doi.org/10.2217/imt.10.32
Bisiaux A, Thiounn N, Timsit MO et al (2009) Molecular analyte profiling of the early events and tissue conditioning following intravesical Bacillus Calmette-Guerin therapy in patients with superficial bladder cancer. J Urol 181:1571–1580. https://doi.org/10.1016/j.juro.2008.11.124
Simons MP, Nauseef WM, Griffith TS (2007) Neutrophils and TRAIL: insights into BCG immunotherapy for bladder cancer. Immunol Res 39:79–93. https://doi.org/10.1007/s12026-007-0084-1
Takayama H, Nishimura K, Tsujimura A et al (2009) Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical Bacillus Calmette-Guerin instillation. J Urol 181:1894–1900. https://doi.org/10.1016/j.juro.2008.11.090
Biot C, Rentsch CA, Gsponer JR et al (2012) Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 4:137ra72. https://doi.org/10.1126/scitranslmed.3003586
Zuiverloon TCM, Nieuweboer AJM, Vékony H et al (2012) Markers predicting response to Bacillus Calmette-Guérin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol 61:128–145. https://doi.org/10.1016/j.eururo.2011.09.026
Riemensberger J, Böhle A, Brandau S (2002) IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol 127:20–26. https://doi.org/10.1046/j.1365-2249.2002.01734.x
Basturk B, Yavascaoglu I, Oral B et al (2006) Cytokine gene polymorphisms can alter the effect of Bacillus Calmette-Guerin (BCG) immunotherapy. Cytokine 35:1–5. https://doi.org/10.1016/j.cyto.2006.06.009
Kucukgergin C, Isman FK, Dasdemir S et al (2012) The role of chemokine and chemokine receptor gene variants on the susceptibility and clinicopathological characteristics of bladder cancer. Gene 511:7–11. https://doi.org/10.1016/j.gene.2012.09.011
Mège J-L, Mehraj V, Capo C (2011) Macrophage polarization and bacterial infections. Curr Opin Infect Dis 24:230–234. https://doi.org/10.1097/QCO.0b013e328344b73e
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795. https://doi.org/10.1172/JCI59643
Pasin E, Josephson DY, Mitra AP et al (2008) Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol 10:31–43. https://doi.org/10.1586/14737140.6.12.1723
Disis ML (2010) Immune regulation of cancer. J Clin Oncol 28:4531–4538. https://doi.org/10.1200/JCO.2009.27.2146
Athie-Morales V, Smits HH, Cantrell DA, Hilkens CMU (2003) Sustained IL-12 signaling is required for Th1 development. J Immunol 172:61–69
Davis ID, Skak K, Smyth MJ et al (2007) Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res 13:6926–6932. https://doi.org/10.1158/1078-0432.CCR-07-1238
Davis MR, Zhu Z, Hansen DM et al (2015) The role of IL-21 in immunity and cancer. Cancer Lett 358:107–114. https://doi.org/10.1016/j.canlet.2014.12.047
Zaidi MR, Merlino G (2011) The two faces of interferon-γ in cancer. Clin Cancer Res 17:6118–6124
Santegoets SJ, Turksma AW, Suhoski MM et al (2013) IL-21 promotes the expansion of CD27+ CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells. J Transl Med 11:37. https://doi.org/10.1186/1479-5876-11-37
Crotty S (2011) Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29:621–663. https://doi.org/10.1146/annurev-immunol-031210-101400
Zhao S, Wu D, Wu P et al (2015) Serum IL-10 predicts worse outcome in cancer patients: a meta-analysis. PLoS One 10:e0139598. https://doi.org/10.1371/journal.pone.0139598
Emmerich J, Mumm JB, Chan IH et al (2012) IL-10 directly activates and expands tumor-resident CD8+ T cells without De Novo infiltration from secondary lymphoid organs. Cancer Res 72:3570–3581. https://doi.org/10.1158/0008-5472.CAN-12-0721
Mumm JB, Oft M (2013) Pegylated IL-10 induces cancer immunity: the surprising role of IL-10 as a potent inducer of IFN-γ-mediated CD8(+) T cell cytotoxicity. Bioessays 35:623–631. https://doi.org/10.1002/bies.201300004
Funding
None.
Author information
Authors and Affiliations
Contributions
GS, TT, and YX conceived and designed the study. GS, HQ, and YX conducted the experiments. GS, TT, and YX analyzed the data. GS and TT wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was reviewed and approved by the Internal Review Board of the Renmin Hospital of Wuhan University (No. RHWU10536).
Informed consent
Informed consents were obtained from all participants in the study.
Rights and permissions
About this article
Cite this article
Shan, G., Tang, T., Qian, H. et al. Certain BCG-reactive responses are associated with bladder cancer prognosis. Cancer Immunol Immunother 67, 797–803 (2018). https://doi.org/10.1007/s00262-018-2127-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2127-y