Abstract
Background
We previously reported overexpression of heat-shock protein (HSP) 70 in hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC) using proteomic profiling and immunohistochemical staining (IHS). This suggested that HSP70 could be a molecular target for treatment of HCC.
Methods
Twelve patients with HCV-related HCC were enrolled in a phase 1 clinical trial. Dendritic cells (DCs) transfected with HSP70 mRNA (HSP70-DCs) induced by electroporation were injected intradermally. Patients were treated three times every 3 weeks. The number of HSP70-DCs injected was 1 × 107 as the lowest dose, then 2 × 107 as the medium dose, and then 3 × 107 as the highest dose. Immunological analyses were performed.
Findings
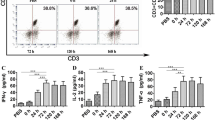
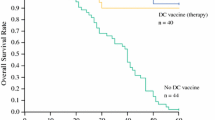
No adverse effects of grade III/IV, except one grade III liver abscess at the 3 × 107 dose, were observed. Thus, we added three more patients to confirm whether 3 × 107 is an appropriate dose. Eventually, we chose 3 × 107 as the recommended dose of DCs. Complete response (CR) without any recurrence occurred in two patients, stable disease in five, and progression of disease in five. The two patients with CR have had no recurrence for 44 and 33 months, respectively. IHS in one patient who underwent partial hepatectomy showed infiltration of CD8+ T cells and granzyme B in tumors, indicating that the dominant immune effector cells were cytotoxic T lymphocytes with tumor-killing activity.
Interpretation
This study demonstrated that HSP70-DCs therapy is both safe and feasible in patients with HCV-related HCC. Further clinical trials should be considered.




Similar content being viewed by others
Abbreviations
- AFP:
-
α-Fetoprotein
- CR:
-
Complete response
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events version 3.0
- CTL:
-
Cytotoxic T cell
- DCs:
-
Dendritic cells
- ECOG PS:
-
Eastern Cooperative Oncology Group Performance Status
- ELISPOT:
-
Enzyme-linked immunospot
- FITC:
-
Fluorescein isothiocyanate
- GM-CFS:
-
Granulocyte–macrophage colony-stimulating factor
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HSP:
-
Heat-shock protein
- HSP70-DCs:
-
HSP70 mRNA-transfected DCs
- IFN-γ:
-
Interferon gamma
- IHS:
-
Immunohistochemical staining
- IL-4:
-
Interleukin-4
- IM-DCs:
-
Immature DCs
- mAb:
-
Monoclonal antibodies
- M-DCs:
-
Mature DCs
- MRI:
-
Magnetic resonance imaging
- PBMCs:
-
Peripheral blood mononuclear cells
- PD:
-
Progression of disease
- PIVKA-II:
-
Vitamin K absence or antagonist II
- RECIST:
-
Response Evaluation Criteria in Solid Tumors guideline version 1.1
- RFA:
-
Radiofrequency ablation
- SD:
-
Stable disease
- SE:
-
Standard error
- TAA:
-
Tumor-associated antigens
- TACE:
-
Transcatheter arterial chemoembolization
- TNF-α:
-
Tumor necrosis factor-α
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Thorgeirsson SS, Grisham JW (2002) Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31(4):339–346. doi:10.1038/ng0802-339
Ohishi W, Kitamoto M, Aikata H, Kamada K, Kawakami Y, Ishihara H, Kamiyasu M, Nakanishi T, Tazuma S, Chayama K (2003) Impact of aging on the development of hepatocellular carcinoma in patients with hepatitis C virus infection in Japan. Scand J Gastroenterol 38(8):894–900
Ikai I, Arii S, Ichida T, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, Yamaoka Y (2005) Report of the 16th follow-up survey of primary liver cancer. Hepatol Res 32(3):163–172. doi:10.1016/j.hepres.2005.04.005
Thomas MB, Abbruzzese JL (2005) Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol 23(31):8093–8108. doi:10.1200/JCO.2004.00.1537
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362(9399):1907–1917. doi:10.1016/S0140-6736(03)14964-1
Iizuka N, Oka M, Yamada-Okabe H, Nishida M, Maeda Y, Mori N, Takao T, Tamesa T, Tangoku A, Tabuchi H, Hamada K, Nakayama H, Ishitsuka H, Miyamoto T, Hirabayashi A, Uchimura S, Hamamoto Y (2003) Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet 361(9361):923–929. doi:10.1016/S0140-6736(03)12775-4
Iizuka N, Hamamoto Y, Oka M (2004) Predicting individual outcomes in hepatocellular carcinoma. Lancet 364(9448):1837–1839. doi:10.1016/S0140-6736(04)17455-2
Itoh Y, Ohkubo K, Iuchi H, Michitaka K, Horiike N, Onji M (2002) Chronological changes of causes of death and distant metastasis in hepatocellular carcinoma. Oncol Rep 9(2):331–335
Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Dono K, Umeshita K, Nakamori S, Wakasa K, Sakon M, Monden M (2007) Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery 141(2):196–202. doi:10.1016/j.surg.2006.06.033
Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ (2006) Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol 45(2):246–253. doi:10.1016/j.jhep.2005.12.027
Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T (2000) Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 356(9232):802–807. doi:10.1016/S0140-6736(00)02654-4
Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, Toda T, Sakaida I, Okita K, Oka M, Nakamura K (2003) Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics 3(12):2487–2493. doi:10.1002/pmic.200300621
Yoshida S, Hazama S, Tokuno K, Sakamoto K, Takashima M, Tamesa T, Torigoe T, Sato N, Oka M (2009) Concomitant overexpression of heat-shock protein 70 and HLA class-I in hepatitis C virus-related hepatocellular carcinoma. Anticancer Res 29(2):539–544
Liao X, Li Y, Bonini C, Nair S, Gilboa E, Greenberg PD, Yee C (2004) Transfection of RNA encoding tumor antigens following maturation of dendritic cells leads to prolonged presentation of antigen and the generation of high-affinity tumor-reactive cytotoxic T lymphocytes. Mol Ther 9(5):757–764. doi:10.1016/j.ymthe.2004.02.011
Boczkowski D, Nair SK, Snyder D, Gilboa E (1996) Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 184(2):465–472
Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN (2001) Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 98(1):49–56
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Tan A, Aucejo F, Kim R (2010) Is there a role for adjuvant treatment after hepatic resection for hepatocellular carcinoma? Oncology 78(3–4):161–171. doi:10.1159/000315577
Litzow MR, Dietz AB, Bulur PA, Butler GW, Gastineau DA, Hoering A, Fink SR, Letendre L, Padley DJ, Paternoster SF, Tefferi A, Vuk-Pavlovic S (2006) Testing the safety of clinical-grade mature autologous myeloid DC in a phase I clinical immunotherapy trial of CML. Cytotherapy 8(3):290–298. doi:10.1080/14653240600735743
Olin MR, Low W, McKenna DH, Haines SJ, Dahlheimer T, Nascene D, Gustafson MP, Dietz AB, Clark HB, Chen W, Blazar B, Ohlfest JR, Moertel C (2014) Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response. J Immunother Cancer 2:4. doi:10.1186/2051-1426-2-4
Xie F, Zhang X, Li H, Zheng T, Xu F, Shen R, Yan L, Yang J, He J (2012) Adoptive immunotherapy in postoperative hepatocellular carcinoma: a systemic review. PLoS One 7(8):e42879. doi:10.1371/journal.pone.0042879
Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS, Onji M (2012) Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol 41(5):1601–1609. doi:10.3892/ijo.2012.1626
Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH (2009) A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49(1):124–132. doi:10.1002/hep.22626
Ferrarini M, Heltai S, Zocchi MR, Rugarli C (1992) Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer 51(4):613–619
Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, Hirohashi S (2003) Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology 37(1):198–207. doi:10.1053/jhep.2003.50022
Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller H, Brunner E, Zientkowska M, Herrmann T, Walter L, Alves F, Multhoff G, Dressel R (2007) The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol 179(8):5523–5533
Takemoto S, Nishikawa M, Guan X, Ohno Y, Yata T, Takakura Y (2010) Enhanced generation of cytotoxic T lymphocytes by heat shock protein 70 fusion proteins harboring both CD8(+) T cell and CD4(+) T cell epitopes. Mol Pharm 7(5):1715–1723. doi:10.1021/mp1001069
Ciocca DR, Cayado-Gutierrez N, Maccioni M, Cuello-Carrion FD (2012) Heat shock proteins (HSPs) based anti-cancer vaccines. Curr Mol Med 12(9):1183–1197
Calderwood SK, Theriault JR, Gong J (2005) Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol 35(9):2518–2527. doi:10.1002/eji.200535002
Zhang HM, Zhang LW, Ren J, Fan L, Si XM, Liu WC (2006) Induction of alpha-fetoprotein-specific CD4- and CD8-mediated T-cell response using RNA-transfected dendritic cells. Cell Immunol 239(2):144–150. doi:10.1016/j.cellimm.2006.05.004
Gilboa E, Vieweg J (2004) Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev 199:251–263. doi:10.1111/j.0105-2896.2004.00139.x
Binder RJ (2009) CD40-independent engagement of mammalian hsp70 by antigen-presenting cells. J Immunol 182(11):6844–6850. doi:10.4049/jimmunol.0900026
Acknowledgments
This study was funded by KAKEN 20591612. We thank Ms. Akiko Sano, Ms. Kaori Kaneyasu, and Ms. Yuko Yanai for their excellent technical assistance.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maeda, Y., Yoshimura, K., Matsui, H. et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol Immunother 64, 1047–1056 (2015). https://doi.org/10.1007/s00262-015-1709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1709-1