Abstract
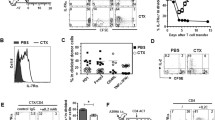
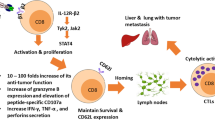
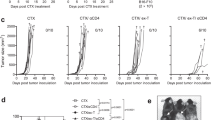
Mouse CD8+ T cells conditioned with interleukin (IL)-12 ex vivo mediate the potent regression of established melanoma when transferred into lymphodepleted mice. However, the quantitative and qualitative changes induced by IL-12 in the responding mouse CD8+ T cells have not been well defined. Moreover, the mechanisms by which IL-12-conditioning impacts human CD8+ T cells, and how such cells might be expanded prior to infusion into patients is not known. We found that ex vivo IL-12-conditioning of mouse CD8+ T cells led to a tenfold–100-fold increase in persistence and anti-tumor efficacy upon adoptive transfer into lymphodepleted mice. The enhancing effect of IL-12 was associated with maintenance of functional avidity. Importantly, in the context of ongoing ACT clinical trials, human CD8+ T cells genetically modified with a tyrosinase-specific T cell receptor (TCR) exhibited significantly enhanced functional activity when conditioned with IL-12 as indicated by heightened granzyme B expression and elevated peptide-specific CD107a degranulation. This effect was sustainable despite the 20 days of in vitro cellular expansion required to expand cells over 1,000-fold allowing adequate cell numbers for administration to cancer patients. Overall, these findings support the efficacy and feasibility of ex vivo IL-12-conditioning of TCR-modified human CD8+ T cells for adoptive transfer and cancer therapy.





Similar content being viewed by others
Abbreviations
- ACT:
-
Adoptive cell therapy
- CAR:
-
Chimeric antigen receptor
- CTX:
-
Cyclophosphamide
- IL-12:
-
Interleukin-12
- REP:
-
Rapid expansion protocol
- Tc0:
-
CD8+ T cells not polarized
- Tc1:
-
CD8+ T cells polarized with IL-12
- TCR:
-
T cell receptor
References
Kershaw MH, Westwood JA, Darcy PK (2013) Gene-engineered T cells for cancer therapy. Nat Rev Cancer 13(8):525–541
Restifo NP, Dudley ME, Rosenberg SA (2012) Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12(4):269–281
Barrett DM, Singh N, Porter DL, Grupp SA, June CH (2014) Chimeric antigen receptor therapy for cancer. Annu Rev Med 65:333–347
Jensen MC, Riddell SR (2014) Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev 257(1):127–144
Muranski P, Restifo NP (2013) Essentials of Th17 cell commitment and plasticity. Blood 121(13):2402–2414
Shrikant PA, Rao R, Li Q, Kesterson J, Eppolito C, Mischo A, Singhal P (2010) Regulating functional cell fates in CD8 T cells. Immunol Res 46(1–3):12–22
Woodland DL, Dutton RW (2003) Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol 15(3):336–342
Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegel JP (1993) Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol 151(5):2444–2452
Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, Familletti PC, Sinigaglia F, Chizonnite R, Gubler U et al (1991) Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol 147(3):874–882
Emtage PC, Clarke D, Gonzalo-Daganzo R, Junghans RP (2003) Generating potent Th1/Tc1 T cell adoptive immunotherapy doses using human IL-12: harnessing the immunomodulatory potential of IL-12 without the in vivo-associated toxicity. J Immunother 26(2):97–106
Halverson DC, Schwartz GN, Carter C, Gress RE, Fowler DH (1997) In vitro generation of allospecific human CD8+ T cells of Tc1 and Tc2 phenotype. Blood 90(5):2089–2096
Sad S, Marcotte R, Mosmann TR (1995) Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2(3):271–279
Croft M, Carter L, Swain SL, Dutton RW (1994) Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med 180(5):1715–1728
Fowler DH, Breglio J, Nagel G, Hirose C, Gress RE (1996) Allospecific CD4+, Th1/Th2 and CD8+, Tc1/Tc2 populations in murine GVL: type I cells generate GVL and type II cells abrogate GVL. Biol Blood Marrow Transplant 2(3):118–125
Dobrzanski MJ, Reome JB, Dutton RW (2000) Type 1 and type 2 CD8+ effector T cell subpopulations promote long-term tumor immunity and protection to progressively growing tumor. J Immunol 164(2):916–925
Dobrzanski MJ, Reome JB, Hollenbaugh JA, Dutton RW (2004) Tc1 and Tc2 effector cell therapy elicit long-term tumor immunity by contrasting mechanisms that result in complementary endogenous type 1 antitumor responses. J Immunol 172(3):1380–1390
Hollenbaugh JA, Dutton RW (2006) IFN-gamma regulates donor CD8 T cell expansion, migration, and leads to apoptosis of cells of a solid tumor. J Immunol 177(5):3004–3011
Helmich BK, Dutton RW (2001) The role of adoptively transferred CD8 T cells and host cells in the control of the growth of the EG7 thymoma: factors that determine the relative effectiveness and homing properties of Tc1 and Tc2 effectors. J Immunol 166(11):6500–6508
Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW (2010) Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol 184(8):4215–4227
Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E, Yu XZ (2013) Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol 190(4):1873–1881
Ye Z, Tang C, Xu S, Zhang B, Zhang X, Moyana T, Yang J, Xiang J (2007) Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell Mol Immunol 4(4):277–285
Curtsinger JM, Mescher MF (2010) Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22(3):333–340
Chang J, Cho JH, Lee SW, Choi SY, Ha SJ, Sung YC (2004) IL-12 priming during in vitro antigenic stimulation changes properties of CD8 T cells and increases generation of effector and memory cells. J Immunol 172(5):2818–2826
Rubinstein MP, Cloud CA, Garrett TE, Moore CJ, Schwartz KM, Johnson CB, Craig DH, Salem ML, Paulos CM, Cole DJ (2012) Ex vivo interleukin-12-priming during CD8(+) T cell activation dramatically improves adoptive T cell transfer antitumor efficacy in a lymphodepleted host. J Am Coll Surg 214(4):700–707; discussion 707–708
Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP (2003) Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 198(4):569–580
Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J (2006) Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 12(3 Pt 1):764–771
Cormier JN, Abati A, Fetsch P, Hijazi YM, Rosenberg SA, Marincola FM, Topalian SL (1998) Comparative analysis of the in vivo expression of tyrosinase, MART-1/Melan-A, and gp100 in metastatic melanoma lesions: implications for immunotherapy. J Immunother 21(1):27–31
Mehrotra S, Al-Khami AA, Klarquist J, Husain S, Naga O, Eby JM, Murali AK, Lyons GE, Li M, Spivey ND, Norell H, Martins da Palma T, Onicescu G, Diaz-Montero CM, Garrett-Mayer E, Cole DJ, Le Poole IC, Nishimura MI (2012) A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. J Immunol 189(4):1627–1638
Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA (2013) Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36(2):133–151
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314(5796):126–129
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA (2012) B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119(12):2709–2720
Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW (2008) IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood 112(9):3704–3712
Hollenbaugh JA, Reome J, Dobrzanski M, Dutton RW (2004) The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol 173(3):1738–1743
Macgregor JN, Li Q, Chang AE, Braun TM, Hughes DP, McDonagh KT (2006) Ex vivo culture with interleukin (IL)-12 improves CD8(+) T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-gamma but requires perforin. Cancer Res 66(9):4913–4921
Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK (1992) IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol 148(10):3125–3132
Szabo SJ, Dighe AS, Gubler U, Murphy KM (1997) Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med 185(5):817–824
Li Q, Eppolito C, Odunsi K, Shrikant PA (2010) Antigen-induced Erk1/2 activation regulates Ets-1-mediated sensitization of CD8+ T cells for IL-12 responses. J Leukoc Biol 87(2):257–263
Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN (1996) Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382(6587):171–174
Kaplan MH, Sun YL, Hoey T, Grusby MJ (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382(6587):174–177
Valenzuela JO, Hammerbeck CD, Mescher MF (2005) Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol 174(2):600–604
Norell H, Zhang Y, McCracken J, Martins da Palma T, Lesher A, Liu Y, Roszkowski JJ, Temple A, Callender GG, Clay T, Orentas R, Guevara-Patino J, Nishimura MI (2010) CD34-based enrichment of genetically engineered human T cells for clinical use results in dramatically enhanced tumor targeting. Cancer Immunol Immunother 59(6):851–862
Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ Jr, Rosenberg SA (2004) Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol 173(12):7125–7130
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R (2005) Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174(10):6477–6489
Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL (1997) Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90(7):2541–2548
Colombo MP, Trinchieri G (2002) Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 13(2):155–168
Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP (2011) IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 121(12):4746–4757
Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, Rosenberg SA, Morgan RA (2011) Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 19(4):751–759
Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ (2012) Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119(18):4133–4141
Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA (2012) Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res 18(6):1672–1683
Acknowledgments
We thank Daniel Neitzke for critical review of this manuscript. Grant funding for this project was provided by the following grants from the National Institutes of Health and the National Cancer Institute: P01CA54778-01, R01CA138930, R01CA133503, and R01CA175061. This work was also supported in part by pilot research funding and also the Cell Evaluation and Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30CA138313).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rubinstein, M.P., Su, E.W., Suriano, S. et al. Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8+ T cells. Cancer Immunol Immunother 64, 539–549 (2015). https://doi.org/10.1007/s00262-015-1655-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1655-y