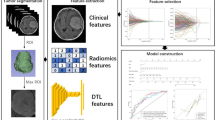
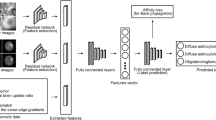
Abstract
Purpose
To explore the value of deep learning-based multi-parametric magnetic resonance imaging (mp-MRI) nomogram in predicting the Ki-67 expression in rectal cancer.
Methods
The data of 491 patients with rectal cancer from two centers were retrospectively analyzed and divided into training, internal validation, and external validation sets. They were categorized into high- and low-expression group based on postoperative pathological Ki-67 expression. Each patient’s mp-MRI data were analyzed to extract and select the most relevant features of deep learning, and a deep learning model was constructed. Independent predictive risk factors were identified and incorporated into a clinical model, and the clinical and deep learning models were combined to obtain a nomogram for the prediction of Ki-67 expression. The performance characteristics of the DL-model, clinical model, and nomogram were assessed using ROCs, calibration curve, decision curve, and clinical impact curve analysis.
Results
The strongest deep learning features were extracted and screened from mp-MRI data. Two independent predictive factors, namely Magnetic Resonance Imaging T (mrT) staging and differentiation degree, were identified through clinical feature selection. Three models were constructed: a deep learning (DL)-model, a clinical model, and a nomogram. The AUCs of clinical model in the training, internal validation, and external validation set were 0.69, 0.78, and 0.67, respectively. The AUCs of the deep model and nomogram ranged from 0.88 to 0.98. The prediction performance of the deep learning model and nomogram was significantly better than the clinical model (P < 0.001).
Conclusion
The nomogram based on deep learning can help clinicians accurately and conveniently predict the expression status of Ki-67 in rectal cancer.
Graphical abstract









Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467-1480.
Cui Y, Liu H, Ren J, Du X, Xin L, Li D, et al. Development and validation of a MRI-based radiomics signature for prediction of KRAS mutation in rectal cancer. Eur Radiol. 2020;30(4):1948-1958.
Mondaca S, Yaeger R. Genetics of rectal cancer and novel therapies: primer for radiologists. Abdom Radiol (NY). 2019;44(11):3743-3750.
Viani L, Dell'Abate P, Del Rio P, Marchesi F, Tartamella F, Rossini M, et al. Colorectal cancer screenings: a single center experience. Acta Biomed. 2020;91(4):e2020101.
Santiago I, Figueiredo N, Parés O, Matos C. MRI of rectal cancer-relevant anatomy and staging key points. Insights Imaging. 2020;11(1):100.
Xie J, Zhao Y, Zhou Y, He Q, Hao H, Qiu X, et al. Predictive Value of Combined Preoperative Carcinoembryonic Antigen Level and Ki-67 Index in Patients With Gastric Neuroendocrine Carcinoma After Radical Surgery. Front Oncol. 2021;11:533039.
Smith I, Robertson J, Kilburn L, Wilcox M, Evans A, Holcombe C, et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020;21(11):1443-1454.
Ao W, Bao X, Mao G, Yang G, Wang J, Hu J. Value of Apparent Diffusion Coefficient for Assessing Preoperative T Staging of Low Rectal Cancer and Whether This Is Correlated With Ki-67 Expression. Can Assoc Radiol J. 2020;71(1):5-11.
Imaizumi K, Suzuki T, Kojima M, Shimomura M, Sakuyama N, Tsukada Y, et al. Ki67 expression and localization of T cells after neoadjuvant therapies as reliable predictive markers in rectal cancer. Cancer Sci. 2020;111(1):23-35.
Tong G, Zhang G, Liu J, Zheng Z, Chen Y, Niu P, et al. Cutoff of 25% for Ki67 expression is a good classification tool for prognosis in colorectal cancer in the AJCC-8 stratification. Oncol Rep. 2020;43(4):1187-1198.
Boros M, Moncea D, Moldovan C, Podoleanu C, Georgescu R, Stolnicu S. Intratumoral Heterogeneity for Ki-67 Index in Invasive Breast Carcinoma: A Study on 131 Consecutive Cases. Appl Immunohistochem Mol Morphol. 2017;25(5):338-340.
Kather JN, Pearson AT, Halama N, Jäger D, Krause J, Loosen SH, et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med. 2019;25(7):1054-1056.
De Paepe KN, Cunningham D. Deep learning as a staging tool in gastric cancer. Ann Oncol. 2020;31(7):827-828.
Liu W, Cheng Y, Liu Z, Liu C, Cattell R, et al. Preoperative Prediction of Ki-67 Status in Breast Cancer with Multiparametric MRI Using Transfer Learning. Acad Radiol. 2021;28(2):e44-e53.
Liang C, Cheng Z, Huang Y, He L, Chen X, Ma Z, et al. An MRI-based Radiomics Classifier for Preoperative Prediction of Ki-67 Status in Breast Cancer. Acad Radiol. 2018;25(9):1111-1117.
Shen L, Zhou G, Tong T, Tang F, Lin Y, Zhou J, et al. ADC at 3.0 T as a noninvasive biomarker for preoperative prediction of Ki67 expression in invasive ductal carcinoma of breast. Clin Imaging. 2018;52:16-22.
Li L, Chen W, Yan Z, Feng J, Hu S, Liu B, et al. Comparative Analysis of Amide Proton Transfer MRI and Diffusion-Weighted Imaging in Assessing p53 and Ki-67 Expression of Rectal Adenocarcinoma. J Magn Reson Imaging. 2020;52(5):1487-1496.
Beets-Tan RG, Beets GL. MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat Rev Gastroenterol Hepatol. 2014;11(8):480-8.
Yao X, Ao W, Zhu X, Tian S, Han X, Hu J, et al. A novel radiomics based on multi-parametric magnetic resonance imaging for predicting Ki-67 expression in rectal cancer: a multicenter study. BMC Med Imaging. 2023;23(1):168.
Yang Y, Li J, Jin L, Wang D, Zhang J, Wang J, et al. Independent Correlation Between Ki67 Index and Circulating Tumor Cells in the Diagnosis of Colorectal Cancer. Anticancer Res. 2017;37(8):4693-4700.
Li P, Xiao ZT, Braciak TA, Ou QJ, Chen G, Oduncu FS. Association between Ki67 Index and Clinicopathological Features in Colorectal Cancer. Oncol Res Treat. 2016;39(11):696-702.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174-83.
Li S, Chen X, Shen K. Association of Ki-67 Change Pattern After Core Needle Biopsy and Prognosis in HR+/HER2- Early Breast Cancer Patients. Front Surg. 2022;9:905575.
Klæstad E, Opdahl S, Raj SX, Bofin AM, Valla M. Long term trends of breast cancer incidence according to proliferation status. BMC Cancer. 2022;22(1):1340.
Vlajnic T, Brunner P, Eppenberger-Castori S, Rentsch CA, Zellweger T, Bubendorf L. High Inter- and Intratumoral Variability of Ki67 Labeling Index in Newly Diagnosed Prostate Cancer with High Gleason Scores. Pathobiology. 2022;89(2):74-80.
Wang L, Liu Z, Fisher KW, Ren F, Lv J, Davidson DD, et al. Prognostic value of programmed death ligand 1, p53, and Ki-67 in patients with advanced-stage colorectal cancer. Hum Pathol. 2018;71:20-29.
Meng X, Li H, Kong L, Zhao X, Huang Z, Zhao H, et al. MRI In rectal cancer: Correlations between MRI features and molecular markers Ki-67, HIF-1α, and VEGF. J Magn Reson Imaging. 2016;44(3):594-600.
Deng S, Ding J, Wang H, Mao G, Sun J, Hu J, et al. Deep learning-based radiomic nomograms for predicting Ki67 expression in prostate cancer. BMC Cancer. 2023;23(1):638.
Li H, Liu Z, Li F, Shi F, Xia Y, Zhou Q, et al. Preoperatively Predicting Ki67 Expression in Pituitary Adenomas Using Deep Segmentation Network and Radiomics Analysis Based on Multiparameter MRI. Acad Radiol. 2023:S1076-6332(23)00278-7.
Lin SL. Application Combining VMD and ResNet101 in Intelligent Diagnosis of Motor Faults. Sensors (Basel). 2021;21(18):6065.
Chen YM, Huang WT, Ho WH, Tsai JT. Classification of age-related macular degeneration using convolutional-neural-network-based transfer learning. BMC Bioinformatics. 2021;22(Suppl 5):99.
You J, Yin J. Performances of Whole Tumor Texture Analysis Based on MRI: Predicting Preoperative T Stage of Rectal Carcinomas. Front Oncol. 2021;11:678441.
Meng X, Xia W, Xie P, Zhang R, Li W, Wang M, et al. Preoperative radiomic signature based on multiparametric magnetic resonance imaging for noninvasive evaluation of biological characteristics in rectal cancer. Eur Radiol. 2019;29(6):3200-3209.
Heijnen LA, Lambregts DM, Mondal D, Martens MH, Riedl RG, Beets GL, et al. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol. 2013;23(12):3354-60.
Su Y, Zhao H, Liu P, Zhang L, Jiao Y, Xu P, et al. A nomogram model based on MRI and radiomic features developed and validated for the evaluation of lymph node metastasis in patients with rectal cancer. Abdom Radiol (NY). 2022;47(12):4103-4114.
Acknowledgements
Not application
Funding
This work was supported by the Medical Science and Technology Project of Zhejiang Province (2022KY122, 2024KY052); Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (2024ZL040).
Author information
Authors and Affiliations
Contributions
SW, WA, and GM contributed to the study conception and design. Material preparation, data collection, and analysis were performed by NW, JH, and WX. The first draft of the manuscript was written by SW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Consent for publication
Not application.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, S., Wang, N., Ao, W. et al. Deep learning-based multi-parametric magnetic resonance imaging (mp-MRI) nomogram for predicting Ki-67 expression in rectal cancer. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04232-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04232-9