Abstract
Purpose
To assess etiological differences in extracellular volume fraction (ECV) and evaluate its influence on staging performance.
Methods
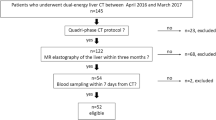
A total of 166 patients with normal liver (n = 14) and chronic liver disease related to viral hepatitis (n = 71), alcohol (n = 44), and nonalcoholic steatohepatitis (NASH) (n = 37) underwent dual-energy CT (DECT) of the liver (5-min equilibrium-phase images) between January 2020 and July 2022. The iodine densities of the parenchyma and aorta were measured and ECV was calculated. Comparisons of ECV between each etiology and METAVIR fibrosis stage were statistically analyzed (p < 0.05).
Results
ECV in each etiology and all patients significantly increased with higher fibrosis stage (p < 0.001) and showed a strong or moderate correlation with fibrosis stage (Spearman’s ρ; all patients, 0.701; viral hepatitis, 0.638; alcoholic, 0.885; NASH, 0.791). In stages F2–F4, ECV in alcoholic liver disease was significantly larger than those for viral hepatitis and NASH (p < 0.05); however, no significant difference in stage F1 was found among the three etiologies. The cutoff values and areas under the receiver operating characteristic curve (AUC-ROCs) for discriminating fibrosis stage (≥ F1– ≥ F4) were higher for alcohol (cutoff values and AUC-ROC; 20.1% and 0.708 for ≥ F1, 23.8% and 0.990 for ≥ F2, 24.3% and 0.968 for ≥ F3, and 26.6% and 0.961 for ≥ F4, respectively) compared with those for the others.
Conclusion
ECV in alcoholic liver disease is higher than that in other etiologies in the advanced stages of fibrosis, and etiological differences in ECV affect the staging performance of fibrosis.
Graphical Abstract







Similar content being viewed by others
References
Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M (2016) Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology 64:1969–1977. https://doi.org/10.1002/hep.28677.
Pinzani M, Rombouts K (2004) Liver fibrosis: from the bench to clinical targets. Dig Liver Dis 36:231–242. https://doi.org/10.1016/j.dld.2004.01.003.
Tsochatzis EA, Bosch J, Burroughs AK (2014) Liver cirrhosis. Lancet 383:1749–1761. https://doi.org/10.1016/S0140-6736(14)60121-5.
Silva AC, Taouli B, Torbenson MS, Wells ML, Yeh B, Miller FH (2017) Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY) 42:2037–2053. https://doi.org/10.1007/s00261-017-1211-7.
Morita K, Nishie A, Ushijima Y, Takayama Y, Fujita N, Kubo Y, Ishimatsu K, Yoshizumi T, Maehara J, Ishigami K (2021) Noninvasive assessment of liver fibrosis by dual-layer spectral detector CT. Eur J Radiol 136:109575. https://doi.org/10.1016/j.ejrad.2021.109575.
Marri UK, Das P, Shalimar, Kalaivani M, Srivastava DN, Madhusudhan KS (2021) Noninvasive Staging of Liver Fibrosis Using 5–Minute Delayed Dual–Energy CT: Comparison with US Elastography and Correlation with Histologic Findings. Radiology 298:600–608. https://doi.org/10.1148/radiol.2021202232.
Ito E, Sato K, Yamamoto R, Sakamoto K, Urakawa H, Yoshimitsu K (2020) Usefulness of iodine-blood material density images in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine dual-energy liver CT protocol equilibrium phase data: preliminary experience. Jpn J Radiol 38:365–373. https://doi.org/10.1007/s11604-019-00918-z.
Sofue K, Tsurusaki M, Mileto A, Hyodo T, Sasaki K, Nishii T, Chikugo T, Yada N, Kudo M, Sugimura K, Murakami T (2018) Dual–energy computed tomography for non–invasive staging of liver fibrosis: Accuracy of iodine density measurements from contrast–enhanced data. Hepatol Res 48:1008–1019. https://doi.org/10.1111/hepr.13205.
Shinagawa Y, Sakamoto K, Sato K, Ito E, Urakawa H, Yoshimitsu K (2018) Usefulness of new subtraction algorithm in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine liver CT protocol equilibrium phase data: Preliminary experience. Eur J Radiol 103:99–104. https://doi.org/10.1016/j.ejrad.2018.04.012.
Guo SL, Su LN, Zhai YN, Chirume WM, Lei JQ, Zhang H, Yang L, Shen XP, Wen XX, Guo YM (2017) The clinical value of hepatic extracellular volume fraction using routine multiphasic contrast–enhanced liver CT for staging liver fibrosis. Clin Radiol 72:242–246. https://doi.org/10.1016/j.crad.2016.10.003.
Bandula S, Punwani S, Rosenberg WM, Jalan R, Hall AR, Dhillon A, Moon JC, Taylor SA (2015) Equilibrium contrast–enhanced CT imaging to evaluate hepatic fibrosis: initial validation by comparison with histopathologic sampling. Radiology 275:136–143. https://doi.org/10.1148/radiol.14141435.
Novo E, Parola M (2008) Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair 1:5. https://doi.org/10.1186/1755-1536-1-5.
Pinzani M (2015) Pathophysiology of Liver Fibrosis. Dig Dis 33:492–497. https://doi.org/10.1159/000374096.
Khatun M, Ray RB (2019) Mechanisms underlying hepatitis C virus-associated hepatic fibrosis. Cells 8:1249. https://doi.org/10.3390/cells8101249.
Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE (2013) From NAFLD to NASH to cirrhosis— new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol 10:627–636. https://doi.org/10.1038/nrgastro.2013.149.
Bian Z, Ma X (2012) Liver fibrogenesis in non-alcoholic steatohepatitis. Front Physiol 3:248. https://doi.org/10.3389/fphys.2012.00248.
Lackner C, Tiniakos D (2012) Fibrosis and alcohol-related liver disease. J Hepatol; 70:294–304. https://doi.org/10.1016/j.jhep.2018.12.003.
Liu SY, Tsai IT, Hsu YC (2021) Alcohol-related liver disease: basic mechanisms and clinical perspectives. Int J Mol Sci 22:5170. https://doi.org/10.3390/ijms22105170.
Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B (2006) Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 130:435–452. https://doi.org/10.1053/j.gastro.2005.10.055.
Jeong WI, Park O, Gao B (2008) Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology 134:248–258. https://doi.org/10.1053/j.gastro.2007.09.034.
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293. https://doi.org/10.1002/hep.510240201.
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22:696–699. https://doi.org/10.1016/0168-8278(95)80226-6.
Kleiner DE, Brunt EM, Van Natta M et al; Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. https://doi.org/10.1002/hep.20701.
Altamirano, J, Miquel, R, Katoonizadeh, A et al (2014) A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 146:1231–1239. https://doi.org/10.1053/j.gastro.2014.01.018.
Ozaki K, Matsui O, Kobayashi S, Minami T, Kitao A, Gabata T (2016) Morphometric changes in liver cirrhosis: aetiological differences correlated with progression. Br J Radiol 89:20150896. https://doi.org/10.1259/bjr.20150896.
Varenika V, Fu Y, Maher JJ, Gao D, Kakar S, Cabarrus MC, Yeh BM (2013) Hepatic fibrosis: evaluation with semiquantitative contrast-enhanced CT. Radiology 266:151–158. https://doi.org/10.1148/radiol.12112452.
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012.
Overholser BR, Sowinski KM. Biostatistics primer: part 2 (2008) Nutr Clin Pract 23:76–84. https://doi.org/10.1177/011542650802300176.
Villeneuve JP, Dagenais M, Huet PM, Roy A, Lapointe R, Marleau D (1996) The hepatic microcirculation in the isolated perfused human liver. Hepatology 23:24–31. https://doi.org/10.1002/hep.510230104.
Faria SC, Ganesan K, Mwangi I, Shiehmorteza M, Viamonte B, Mazhar S, Peterson M, Kono Y, Santillan C, Casola G, Sirlin CB (2009) MR imaging of liver fibrosis: current state of the art. Radiographics 29:1615–1635. https://doi.org/10.1148/rg.296095512.
Ozaki K, Kozaka K, Kosaka Y, Kimura H, Gabata T (2020) Morphometric changes and imaging findings of diffuse liver disease in relation to intrahepatic hemodynamics. Jpn J Radiol 38:833–852. https://doi.org/10.1007/s11604-020-00978-6.
Maher JJ (1990) Hepatic fibrosis caused by alcohol. Semin Liver Dis 10:66–74. https://doi.org/10.1055/s-2008-1040458.
Vizzotto L, Vertemati M, Gambacorta M, Sabatella G, Spina V, Minola E (2002) Analysis of histological and immunohistochemical patterns of the liver in posthepatitic and alcoholic cirrhosis by computerized morphometry. Mod Pathol 15:798–806. https://doi.org/10.1097/01.MP.0000024365.92937.5E.
Bak S, Kim JE, Bae K, Cho JM, Choi HC, Park MJ, Choi HY, Shin HS, Lee SM, Kim HO (2020) Quantification of liver extracellular volume using dual-energy CT: utility for prediction of liver-related events in cirrhosis. Eur Radiol 30:5317–5326. https://doi.org/10.1007/s00330-020-06876-9.
European Association for the Study of Liver (2012) EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 57:399–420. https://doi.org/10.1016/j.jhep.2012.04.004.
Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH (2018) ACG Clinical Guideline: Alcoholic liver disease. Am J Gastroenterol 113:175–194. https://doi.org/10.1038/ajg.2017.469.
Ozaki K, Ishida T, Ohtani T, Shimada M, Kimura H, Gabata T (2021) Assessing the progression of segmental fibrosis in chronic liver disease using extracellular volume fractions Eur J Radiol 145:110033. https://doi.org/10.1016/j.ejrad.2021.110033.
Marin D, Pratts-Emanuelli JJ, Mileto A, Husarik DB, Bashir MR, Nelson RC, Boll DT (2015) Interdependencies of acquisition, detection, and reconstruction techniques on the accuracy of iodine quantification in varying patient sizes employing dual-energy CT. Eur Radiol 25:679–686. https://doi.org/10.1007/s00330-014-3447-8.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
KO: conceptualization; KO, TI: methodology; TO, SI, SH, TI, KT, YM: data curation and investigation; SI: formal analysis; SH: visualization; KO, TO: writing—original draft preparation; SI, HK, TG: writing—review and editing; TG: supervision.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
This single-center retrospective study was approved by our institution’s Research Ethics Committee (approval No.: 20190164). Informed consent for contrast-enhanced CT was obtained from all patients before the examinations.
Research involving human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozaki, K., Ohtani, T., Ishida, S. et al. Extracellular volume fraction obtained by dual-energy CT depicting the etiological differences of liver fibrosis. Abdom Radiol 48, 1975–1986 (2023). https://doi.org/10.1007/s00261-023-03873-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03873-6