Abstract
Purpose
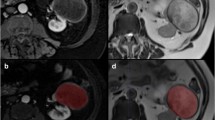
Solid renal masses (SRM) are difficult to differentiate based on standard MR features. The purpose of this study was to assess MR imaging features of SRM to evaluate performance of ensemble methods of classifying SRM subtypes.
Materials and methods
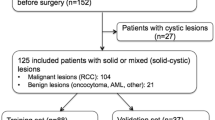
MR images of SRM (n = 330) were retrospectively evaluated for standard and multiparametric (mp) features. Models of MR features for predicting malignant and benign lesions as well as subtyping SRM were developed using a training dataset and performance was evaluated in a test data-set using recursive partitioning (RP), gradient booting machine (GBM), and random forest (RF) methods.
Results
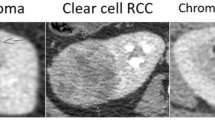
In the test dataset, GBM and RF models demonstrated an accuracy of 86% (95% CI 75% to 93%) for predicting benign versus malignant SRM compared to 83% (95% CI 71% to 91%) for the RP model. RF had the greatest accuracy in predicting SRM subtypes, 81.2% (95% CI 69.5% to 89.9%) compared with GBM 73.4% (95% CI 60.9% to 83.7%) or RP 70.3% (95% CI 57.6% to 81.1%). Marginal homogeneity was reduced by the RF model compared with the RP model (P < 0.001), but not the GBM model (P = 0.135). All models had high sensitivity and specificity for clear cell and papillary renal cell carcinomas (RCC), but performed less well in differentiating chromophobe RCC, oncocytomas, and fat-poor angiomyolipomas.
Conclusion
Ensemble methods for prediction of SRM from radiologist-assessed image characteristics have high accuracy for distinguishing benign and malignant lesions. SRM subtype classification is limited by the ability to categorize chromophobe RCCs, oncocytomas, and fat-poor angiomyolipomas.




Similar content being viewed by others
References
1. Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69 (1):7-34. https://doi.org/10.3322/caac.21551
Kay FU, Canvasser NE, Xi Y, Pinho DF, Costa DN, Diaz de Leon A, Khatri G, Leyendecker JR, Yokoo T, Lay AH, Kavoussi N, Koseoglu E, Cadeddu JA, Pedrosa I (2018) Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology:171557. https://doi.org/10.1148/radiol.2018171557
3. Muglia VF, Prando A (2015) Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras 48 (3):166-174. https://doi.org/10.1590/0100-3984.2013.1927
4. Lopes Vendrami C, Parada Villavicencio C, DeJulio TJ, Chatterjee A, Casalino DD, Horowitz JM, Oberlin DT, Yang GY, Nikolaidis P, Miller FH (2017) Differentiation of solid renal tumors with multiparametric MR imaging. Radiographics 37 (7):2026-2042. https://doi.org/10.1148/rg.2017170039
5. Lin F, Cui EM, Lei Y, Luo LP (2019) CT-based machine learning model to predict the Fuhrman nuclear grade of clear cell renal cell carcinoma. Abdom Radiol (NY) 44 (7):2528-2534. https://doi.org/10.1007/s00261-019-01992-7
6. Motzer RJ, Agarwal N, Beard C, Bhayani S, Bolger GB, Carducci MA, Chang SS, Choueiri TK, Hancock SL, Hudes GR, Jonasch E, Josephson D, Kuzel TM, Levine EG, Lin DW, Margolin KA, Michaelson MD, Olencki T, Pili R, Ratliff TW, Redman BG, Robertson CN, Ryan CJ, Sheinfeld J, Spiess PE, Wang J, Wilder RB, National Comprehensive Cancer N (2011) Kidney cancer. J Natl Compr Canc Netw 9 (9):960-977
7. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol 70 (1):93-105. https://doi.org/10.1016/j.eururo.2016.02.029
8. Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27 (5):612-624
9. Ramamurthy NK, Moosavi B, McInnes MD, Flood TA, Schieda N (2015) Multiparametric MRI of solid renal masses: pearls and pitfalls. Clin Radiol 70 (3):304-316. https://doi.org/10.1016/j.crad.2014.10.006
10. Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Guille F, Chopin DK, Mulders PF, Wood CG, Swanson DA, Figlin RA, Belldegrun AS, Pantuck AJ (2005) Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol 23 (12):2763-2771. https://doi.org/10.1200/jco.2005.07.055
11. Campbell N, Rosenkrantz AB, Pedrosa I (2014) MRI phenotype in renal cancer: is it clinically relevant? Top Magn Reson Imaging 23 (2):95-115. https://doi.org/10.1097/rmr.0000000000000019
12. Vendrami CL, Velichko YS, Miller FH, Chatterjee A, Villavicencio CP, Yaghmai V, McCarthy RJ (2018) Differentiation of papillary renal cell carcinoma subtypes on MRI: qualitative and texture analysis. AJR Am J Roentgenol 211 (6):1234-1245. https://doi.org/10.2214/ajr.17.19213
13. Casuscelli J, Becerra MF, Seier K, Manley BJ, Benfante N, Redzematovic A, Stief CG, Hsieh JJ, Tickoo SK, Reuter VE, Coleman JA, Russo P, Ostrovnaya I, Hakimi AA (2019) Chromophobe renal cell carcinoma: results from a large single-institution series. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2019.06.011
14. Low G, Huang G, Fu W, Moloo Z, Girgis S (2016) Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 8 (5):484-500. https://doi.org/10.4329/wjr.v8.i5.484
15. Kay FU, Pedrosa I (2017) Imaging of solid renal masses. Radiol Clin North Am 55 (2):243-258. https://doi.org/10.1016/j.rcl.2016.10.003
16. Rosenkrantz AB, Hindman N, Fitzgerald EF, Niver BE, Melamed J, Babb JS (2010) MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AJR Am J Roentgenol 195 (6):W421-427. https://doi.org/10.2214/ajr.10.4718
17. Cornelis F, Tricaud E, Lasserre AS, Petitpierre F, Bernhard JC, Le Bras Y, Yacoub M, Bouzgarrou M, Ravaud A, Grenier N (2014) Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours. Eur Radiol 24 (5):1068-1080. https://doi.org/10.1007/s00330-014-3107-z
18. Cornelis F, Grenier N (2017) Multiparametric magnetic resonance imaging of solid renal tumors: a practical algorithm. Semin Ultrasound CT MR 38 (1):47-58. https://doi.org/10.1053/j.sult.2016.08.009
19. Akin IB, Altay C, Guler E, Camlidag I, Harman M, Danaci M, Tuna B, Yorukoglu K, Secil M (2019) Discrimination of oncocytoma and chromophobe renal cell carcinoma using MRI. Diagn Interv Radiol 25 (1):5-13. https://doi.org/10.5152/dir.2018.18013
20. Strobl C, Malley J, Tutz G (2009) An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods 14 (4):323-348. https://doi.org/10.1037/a0016973
21. Friedman J, Hastie T, Tibshirani R (2000) Additive logistic regression: a statistical view of boosting. The Annals of Statistics 28 (2):337-407. https://doi.org/10.1214/aos/1016218223
22. Breiman L (2001) Random forests. Machine Learning 45 (October):5-32. doi:https://doi.org/10.1023/A:1010933404324
23. Oliva MR, Glickman JN, Zou KH, Teo SY, Mortele KJ, Rocha MS, Silverman SG (2009) Renal cell carcinoma: t1 and t2 signal intensity characteristics of papillary and clear cell types correlated with pathology. AJR Am J Roentgenol 192 (6):1524-1530. https://doi.org/10.2214/ajr.08.1727
24. Hotker AM, Mazaheri Y, Wibmer A, Karlo CA, Zheng J, Moskowitz CS, Tickoo SK, Russo P, Hricak H, Akin O (2017) Differentiation of clear cell renal cell carcinoma from other renal cortical tumors by use of a quantitative multiparametric MRI approach. AJR Am J Roentgenol 208 (3):W85-W91. https://doi.org/10.2214/ajr.16.16652
25. Pedrosa I, Sun MR, Spencer M, Genega EM, Olumi AF, Dewolf WC, Rofsky NM (2008) MR imaging of renal masses: correlation with findings at surgery and pathologic analysis. Radiographics 28 (4):985-1003. https://doi.org/10.1148/rg.284065018
26. Gurel S, Narra V, Elsayes KM, Siegel CL, Chen ZE, Brown JJ (2013) Subtypes of renal cell carcinoma: MRI and pathological features. Diagn Interv Radiol 19 (4):304-311. https://doi.org/10.5152/dir.2013.147
Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, Dalrymple NC, Chintapalli KN (2006) Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics 26 (6):1795-1806; discussion 1806-1710. https://doi.org/10.1148/rg.266065010
28. Rosenkrantz AB, Sekhar A, Genega EM, Melamed J, Babb JS, Patel AD, Lo A, Najarian RM, Ahmed M, Pedrosa I (2013) Prognostic implications of the magnetic resonance imaging appearance in papillary renal cell carcinoma. Eur Radiol 23 (2):579-587. https://doi.org/10.1007/s00330-012-2631-y
29. Galmiche C, Bernhard JC, Yacoub M, Ravaud A, Grenier N, Cornelis F (2017) Is multiparametric MRI useful for differentiating oncocytomas from chromophobe renal cell carcinomas? AJR Am J Roentgenol 208 (2):343-350. https://doi.org/10.2214/ajr.16.16832
30. Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M (2014) Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging 39 (3):588-604. https://doi.org/10.1007/s00261-014-0083-3
31. Israel GM, Hindman N, Hecht E, Krinsky G (2005) The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR Am J Roentgenol 184 (6):1868-1872. https://doi.org/10.2214/ajr.184.6.01841868
32. Hindman N, Ngo L, Genega EM, Melamed J, Wei J, Braza JM, Rofsky NM, Pedrosa I (2012) Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology 265 (2):468-477. https://doi.org/10.1148/radiol.12112087
33. Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A (2012) Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology 263 (1):160-168. https://doi.org/10.1148/radiol.12111205
34. Hotker AM, Mazaheri Y, Wibmer A, Zheng J, Moskowitz CS, Tickoo SK, Russo P, Hricak H, Akin O (2016) Use of DWI in the differentiation of renal cortical tumors. AJR Am J Roentgenol 206 (1):100-105. https://doi.org/10.2214/ajr.14.13923
35. Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A, Iyer VK, Das P (2012) Diffusion-weighted MRI in renal cell carcinoma: a surrogate marker for predicting nuclear grade and histological subtype. Acta Radiol 53 (3):349-358. https://doi.org/10.1258/ar.2011.110415
Wang H, Cheng L, Zhang X, Wang D, Guo A, Gao Y, Ye H (2010) Renal cell carcinoma: diffusion-weighted MR imaging for subtype differentiation at 3.0 T. Radiology 257 (1):135-143. https://doi.org/10.1148/radiol.10092396
37. Sandrasegaran K, Sundaram CP, Ramaswamy R, Akisik FM, Rydberg MP, Lin C, Aisen AM (2010) Usefulness of diffusion-weighted imaging in the evaluation of renal masses. AJR Am J Roentgenol 194 (2):438-445. https://doi.org/10.2214/ajr.09.3024
38. Choi YA, Kim CK, Park SY, Cho SW, Park BK (2014) Subtype differentiation of renal cell carcinoma using diffusion-weighted and blood oxygenation level-dependent MRI. AJR Am J Roentgenol 203 (1):W78-84. https://doi.org/10.2214/ajr.13.11551
Yu X, Lin M, Ouyang H, Zhou C, Zhang H (2012) Application of ADC measurement in characterization of renal cell carcinomas with different pathological types and grades by 3.0T diffusion-weighted MRI. Eur J Radiol 81 (11):3061-3066. https://doi.org/10.1016/j.ejrad.2012.04.028
40. Taouli B, Thakur RK, Mannelli L, Babb JS, Kim S, Hecht EM, Lee VS, Israel GM (2009) Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology 251 (2):398-407. https://doi.org/10.1148/radiol.2512080880
41. Mytsyk Y, Dutka I, Borys Y, Komnatska I, Shatynska-Mytsyk I, Farooqi AA, Gazdikova K, Caprnda M, Rodrigo L, Kruzliak P (2017) Renal cell carcinoma: applicability of the apparent coefficient of the diffusion-weighted estimated by MRI for improving their differential diagnosis, histologic subtyping, and differentiation grade. Int Urol Nephrol 49 (2):215-224. https://doi.org/10.1007/s11255-016-1460-3
42. Mytsyk Y, Dutka I, Yuriy B, Maksymovych I, Caprnda M, Gazdikova K, Rodrigo L, Kruzliak P, Illjuk P, Farooqi AA (2018) Differential diagnosis of the small renal masses: role of the apparent diffusion coefficient of the diffusion-weighted MRI. Int Urol Nephrol 50 (2):197-204. https://doi.org/10.1007/s11255-017-1761-1
43. Abdel Razek AA, Mousa A, Farouk A, Nabil N (2016) Assessment of semiquantitative parameters of dynamic contrast-enhanced perfusion MR imaging in differentiation of subtypes of renal cell carcinoma. Pol J Radiol 81:90-94. https://doi.org/10.12659/pjr.894707
44. Vargas HA, Chaim J, Lefkowitz RA, Lakhman Y, Zheng J, Moskowitz CS, Sohn MJ, Schwartz LH, Russo P, Akin O (2012) Renal cortical tumors: use of multiphasic contrast-enhanced MR imaging to differentiate benign and malignant histologic subtypes. Radiology 264 (3):779-788. https://doi.org/10.1148/radiol.12110746
45. Kunapuli G, Varghese BA, Ganapathy P, Desai B, Cen S, Aron M, Gill I, Duddalwar V (2018) A decision-support tool for renal mass classification. J Digit Imaging 31 (6):929-939. https://doi.org/10.1007/s10278-018-0100-0
Tanaka T, Huang Y, Marukawa Y, Tsuboi Y, Masaoka Y, Kojima K, Iguchi T, Hiraki T, Gobara H, Yanai H, Nasu Y, Kanazawa S (2020) Differentiation of small (</= 4 cm) renal masses on multiphase contrast-enhanced CT by deep learning. AJR Am J Roentgenol:1-8. https://doi.org/10.2214/ajr.19.22074
47. Sun XY, Feng QX, Xu X, Zhang J, Zhu FP, Yang YH, Zhang YD (2020) Radiologic-radiomic machine learning models for differentiation of benign and malignant solid renal masses: comparison with expert-level radiologists. AJR Am J Roentgenol 214 (1):W44-W54. https://doi.org/10.2214/ajr.19.21617
Funding
No funding or grant support was used in the preparing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopes Vendrami, C., McCarthy, R.J., Villavicencio, C.P. et al. Predicting common solid renal tumors using machine learning models of classification of radiologist-assessed magnetic resonance characteristics. Abdom Radiol 45, 2797–2809 (2020). https://doi.org/10.1007/s00261-020-02637-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02637-w