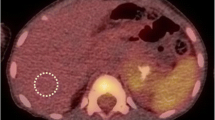
Abstract
Purpose
Iodine 123 labeled meta-iodobenzylguanidine ([123I]MIBG) scan with SPECT/CT imaging is one of the most commonly used imaging modalities in the evaluation of neuroblastoma. [18F]-meta-fluorobenzylguanidine ([18F]MFBG) is a novel positron emission tomography (PET) tracer which was reported to have a similar biodistribution to [123I]MIBG. However, the experience of using [18F]MFBG PET/CT in the evaluation of patients with neuroblastoma is limited. This preliminary investigation aims to assess the efficacy of [18F]MFBG PET/CT in the evaluation of neuroblastomas in comparison to [123I]MIBG scans with SPECT/CT.
Materials and methods
In this prospective, single-center study, 40 participants (mean age 6.0 ± 3.7 years) with history of neuroblastoma were enrolled. All children underwent both [123I]MIBG SPECT/CT and [18F]MFBG PET/CT studies. The number of lesions and the Curie scores revealed by each imaging method were recorded.
Results
Six patients had negative findings on both [123I]MIBG and [18F]MFBG studies. Four of the 34 patients (11.8%) were negative on [123I]MIBG but positive on [18F]MFBG, while 30 patients were positive on both [123I]MIBG and [18F]MFBG studies. In these 34 patients, [18F]MFBG PET/CT identified 784 lesions while [123I]MIBG SPECT/CT detected 532 lesions (p < 0.001). The Curie scores obtained from [18F]MFBG PET/CT (11.32 ± 8.18, range 1-27) were statistically higher (p < 0.001) than those from [123I]MIBG SPECT/CT (7.74 ± 7.52, range 0-26). 30 of 34 patients (88.2%) with active disease on imaging had higher Curie scores based on the [18F]MFBG study than on the [123I]MIBG imaging.
Conclusion
[18F]MFBG PET/CT shows higher lesion detection rate than [123I]MIBG SPECT/CT in the evaluation of pediatric patients with neuroblastoma.
Clinical trial registration
Clinicaltrials.gov: NCT05069220 (Registered: 25 September 2021, retrospectively registered); Institute Review Board of Peking Union Medical College Hospital: ZS-2514





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Berthold F, Spix C, Kaatsch P, Lampert F. Incidence, Survival, and Treatment of Localized and Metastatic Neuroblastoma in Germany 1979-2015. Paediatr Drugs. 2017;19(6):577–93.
Bosse KR, Maris JM. Advances in the translational genomics of neuroblastoma: From improving risk stratification and revealing novel biology to identifying actionable genomic alterations. Cancer. 2016;122(1):20–33.
Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol. 2015;33(27):3008–17.
Tas ML, Reedijk AMJ, Karim-Kos HE, et al. Neuroblastoma between 1990 and 2014 in the Netherlands: Increased incidence and improved survival of high-risk neuroblastoma. Eur J Cancer. 2020;124:12447–55.
Morgenstern DA, London WB, Stephens D, et al. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: A study from the International Neuroblastoma Risk Group database. Eur J Cancer. 2016;65:1–10.
Pandit-Taskar N, Modak S. Norepinephrine Transporter as a Target for Imaging and Therapy. J Nucl Med. 2017;58(Suppl 2):39S–53S.
Liu B, Zhuang H, Servaes S. Comparison of [123I]MIBG and [131I]MIBG for imaging of neuroblastoma and other neural crest tumors. Q J Nucl Med Mol Imaging. 2013;57(1):21–8.
Liu B, Servaes S, Zhuang H. SPECT/CT MIBG Imaging Is Crucial in the Follow-up of the Patients With High-Risk Neuroblastoma. Clin Nucl Med. 2018;43(4):232–8.
Naranjo A, Parisi MT, Shulkin BL, et al. Comparison of (1)(2)(3)I-metaiodobenzylguanidine (MIBG) and (1)(3)(1)I-MIBG semi-quantitative scores in predicting survival in patients with stage 4 neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;56(7):1041–5.
Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children's oncology group. J Nucl Med. 2013;54(4):541–8.
Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27(2):298–303.
Park JR, Bagatell R, Cohn SL, et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2017;35(22):2580–7.
Bar-Sever Z, Biassoni L, Shulkin B, et al. Guidelines on nuclear medicine imaging in neuroblastoma. Eur J Nucl Med Mol Imaging. 2018;45(11):2009–24.
Wen Z, Zhang L, Zhuang H. Roles of PET/Computed Tomography in the Evaluation of Neuroblastoma. PET Clin. 2020;15(3):321–31.
Huang SY, Bolch WE, Lee C, et al. Patient-specific dosimetry using pretherapy [124I]m-iodobenzylguanidine ([124I]mIBG) dynamic PET/CT imaging before [131I]mIBG targeted radionuclide therapy for neuroblastoma. Mol Imaging Biol. 2015;17(2):284–94.
Aboian MS, Huang SY, Hernandez-Pampaloni M, et al. (124)I-MIBG PET/CT to Monitor Metastatic Disease in Children with Relapsed Neuroblastoma. J Nucl Med. 2021;62(1):43–7.
Pentlow KS, Graham MC, Lambrecht RM, et al. Quantitative imaging of iodine-124 with PET. J Nucl Med. 1996;37(9):1557–62.
Lee CL, Wahnishe H, Sayre GA, et al. Radiation dose estimation using preclinical imaging with 124I-metaiodobenzylguanidine (MIBG) PET. Med Phys. 2010;37(9):4861–7.
Zhang H, Huang R, Cheung NK, et al. Imaging the norepinephrine transporter in neuroblastoma: a comparison of [18F]-MFBG and 123I-MIBG. Clin Cancer Res. 2014;20(8):2182–91.
Pandit-Taskar N, Zanzonico P, Staton KD, et al. Biodistribution and Dosimetry of (18)F-Meta-Fluorobenzylguanidine: A First-in-Human PET/CT Imaging Study of Patients with Neuroendocrine Malignancies. J Nucl Med. 2018;59(1):147–53.
Samim A, Blom T, Poot AJ, et al. [(18)F]mFBG PET-CT for detection and localisation of neuroblastoma: a prospective pilot study. Eur J Nucl Med Mol Imaging. 2023;50(4):1146–57.
Kinuya S, Yoshinaga K, Higuchi T, et al. Draft guidelines regarding appropriate use of (131)I-MIBG radiotherapy for neuroendocrine tumors : Guideline Drafting Committee for Radiotherapy with (131)I-MIBG, Committee for Nuclear Oncology and Immunology, The Japanese Society of Nuclear Medicine. Ann Nucl Med. 2015;29(6):543–52.
Bombardieri E, Giammarile F, Aktolun C, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37(12):2436–46.
Turnock S, Turton DR, Martins CD, et al. (18)F-meta-fluorobenzylguanidine ((18)F-mFBG) to monitor changes in norepinephrine transporter expression in response to therapeutic intervention in neuroblastoma models. Sci Rep. 2020;10(1):20918.
Bai X, Wang X, Zhuang H. Increased Gastric MIBG Activity as a Normal Variant. Clin Nucl Med. 2019;44(9):761–3.
Wang W, Yang X, Yang J. Elevated 123I-MIBG Activity in Intramuscular Hemangioma. Clin Nucl Med. 2021;46(2):168–70.
Bai X, Zhuang H. Focally Increased MIBG Activity in the Muscle: Real Lesion or LOVENOX Injection Artifact? Clin Nucl Med. 2016;41(2):167–8.
Yang J, Codreanu I, Servaes S, Zhuang H. Persistent intense MIBG activity in the liver caused by prior radiation. Clin Nucl Med. 2014;39(10):926–30.
Schindler T, Yu C, Rossleigh M, et al. False-positive MIBG uptake in pneumonia in a patient with stage IV neuroblastoma. Clin Nucl Med. 2010;35(9):743–5.
Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77.
Irwin MS, Naranjo A, Zhang FF, et al. Revised Neuroblastoma Risk Classification System: A Report From the Children's Oncology Group. J Clin Oncol. 2021;39(29):3229–41.
Samim A, Tytgat GAM, Bleeker G, et al. Nuclear Medicine Imaging in Neuroblastoma: Current Status and New Developments. J Pers Med. 2021;11(4):270.
Pauwels E, Celen S, Baete K, et al. [(18)F] MFBG PET imaging: biodistribution, pharmacokinetics, and comparison with [(123)I] MIBG in neural crest tumour patients. Eur J Nucl Med Mol Imaging. 2023;50(4):1134–45.
Zhang H, Huang R, Pillarsetty N, et al. Synthesis and evaluation of 18F-labeled benzylguanidine analogs for targeting the human norepinephrine transporter. Eur J Nucl Med Mol Imaging. 2014;41(2):322–32.
Wang P, Li T, Cui Y, et al. 18 F-MFBG PET/CT Is an Effective Alternative of 68 Ga-DOTATATE PET/CT in the Evaluation of Metastatic Pheochromocytoma and Paraganglioma. Clin Nucl Med. 2023;48(1):43–8.
Wang P, Hou G, Jing H, et al. Similar Findings on 18F-MFBG PET/CT and 68Ga-DOTATATE PET/CT in a Patient With Widespread Metastatic Pheochromocytoma. Clin Nucl Med. 2022;47(5):451–3.
Pauwels E, Celen S, Vandamme M, et al. Improved resolution and sensitivity of [(18)F]MFBG PET compared with [(123)I]MIBG SPECT in a patient with a norepinephrine transporter-expressing tumour. Eur J Nucl Med Mol Imaging. 2021;48(1):313–5.
Deng M, Shu Q, Hu M, et al. Comparison of 18 F-MFBG and 68 Ga-DOTATATE PET/CT in the Imaging of Metastatic Paraganglioma and Pheochromocytoma. Clin Nucl Med. 2022;47(12):e735–e7.
Suurd DPD, Poot AJ, van Leeuwaarde RS, et al. [(18)F]mFBG PET/CT imaging outperforms MRI and [(68) Ga]Ga-DOTA-TOC PET/CT in identifying recurrence pheochromocytoma. Eur J Nucl Med Mol Imaging. 2023;50(5):1538–40.
Funding
This work was supported by Chinese Academy of Medical Science (CAMS) Innovation Fund for Medical Sciences (grant number: CF-2021-12M-1-2-2022564), National High Level Hospital Clinical Research Funding (grant number: 2022-PUMCH-C-003), and National High Level Hospital Clinical Research Funding (grant number: 2022-PUMCH-B-070).
Author information
Authors and Affiliations
Contributions
Guarantors of the integrity of the entire study, all authors; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of the final version of the submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, P.W., Z.L., M.J., F.L.; clinical studies, P.W., T.L., Z.L., M.J., Y.S., H.J., F.L.; experimental studies, P.W., Z.L., M.J., Y.S.; data analysis, P.W., T.L., H.J., J.Z., H.Z., F.L.; and manuscript editing, P.W., T.L., J.Z., Y.S., H.J., H.Z., F.L.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Peking Union Medical College Hospital (Date: 27-Jul-2021/No: ZS-2514).
Consent to participate
Written informed consent was obtained from guardians of patient. In addition, when patients were over the age of 8, consent from the patients were also obtained.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figure(s) 2, 3, 4.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Li, T., Liu, Z. et al. [18F]MFBG PET/CT outperforming [123I]MIBG SPECT/CT in the evaluation of neuroblastoma. Eur J Nucl Med Mol Imaging 50, 3097–3106 (2023). https://doi.org/10.1007/s00259-023-06221-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06221-4