Abstract
Purpose
According to IAEA/EANM/SNMMI guidelines, long-acting somatostatin analogues (LA-SSAs) should be discontinued 4–6 weeks prior to peptide receptor radionuclide therapy (PRRT) to prevent somatostatin receptor saturation. The aim of this study was to determine the effect of continued use of long-acting SSAs during PRRT on the uptake of [177Lu]Lu-HA-DOTATATE on SPECT/CT.
Methods
Consecutive patients with neuroendocrine tumours who were treated with PRRT receiving 7.4 GBq of [177Lu]Lu-HA-DOTATATE were included. Patients were divided into 3 groups: (1) control (LA-SSA stopped > 6 weeks prior to PRRT), or continued treatment with (2) long-acting octreotide < 6 weeks prior to PRRT, or (3) long-acting lanreotide < 6 weeks prior to PRRT. The uptake of [177Lu]Lu-HA-DOTATATE was quantified in healthy tissues (spleen, liver, kidneys, bone marrow) and tumour lesions on SPECT/CT performed 24 h after PRRT. A Mann–Whitney U test was used to determine differences in uptake between the long-acting octreotide and long-acting lanreotide groups compared to the control group.
Results
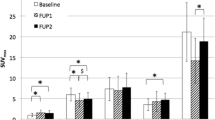
Forty-two patients with 135 cycles of PRRT were included: 28 with lanreotide, 50 with octreotide, and 57 cycles without LA-SSAs. Uptake of [177Lu]Lu-HA-DOTATATE was significantly decreased in liver parenchyma in patients with lanreotide (p < 0.001) and in the spleen in patients with either octreotide or lanreotide (both p < 0.001). No differences were observed for uptake in kidneys, bone marrow, and blood pool. Uptake of [177Lu]Lu-HA-DOTATATE in tumours was the same in patients with lanreotide compared to the control (p = 0.862) and in patients with octreotide compared to the control (p = 0.201), independent of tumour location.
Conclusion
Long-acting octreotide and lanreotide do not interfere with the uptake of [177Lu]Lu-HA-DOTATATE in tumour lesions 24 h post-injection. Uptake in healthy liver parenchyma significantly decreases after lanreotide administration prior to PRRT, while uptake in healthy spleen tissue significantly decreases with both octreotide and lanreotide administration.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42.
Salyers WJ, Vega KJ, Munoz JC, Trotman BW, Tanev SS. Neuroendocrine tumors of the gastrointestinal tract: case reports and literature review. World J Gastrointest Oncol. 2014;6(8):301–10.
Stueven AK, Kayser A, Wetz C, Amthauer H, Wree A, Tacke F, et al. Somatostatin analogues in the treatment of neuroendocrine tumors: past, present and future. Int J Mol Sci. 2019;20(12):3049.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–35.
Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, et al. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47(3):502–11.
Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40(5):800–16.
Aalbersberg EA, de Wit-van der Veen BJ, Versleijen MWJ, Saveur LJ, Valk GD, Tesselaar MET, et al. Influence of lanreotide on uptake of (68)Ga-DOTATATE in patients with neuroendocrine tumours: a prospective intra-patient evaluation. Eur J Nucl Med Mol Imaging. 2019;46(3):696–703.
Cherk MH, Kong G, Hicks RJ, Hofman MS. Changes in biodistribution on 68Ga-DOTA-Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging. 2018;18(1):3.
Lodge MA, Solnes LB, Chaudhry MA, Wahl RL. Prospective within-patient assessment of the impact of an unlabeled octreotide pre-dose on the biodistribution and tumor uptake of (68)Ga DOTATOC as assessed by dynamic whole-body PET in patients with neuroendocrine tumors: implications for diagnosis and therapy. Mol Imaging Biol. 2021;23(5):766–74.
Gålne A, Almquist H, Almquist M, Hindorf C, Ohlsson T, Nordenström E, et al. A prospective observational study to evaluate the effects of long-acting somatostatin analogs on (68)Ga-DOTATATE uptake in patients with neuroendocrine tumors. J Nucl Med. 2019;60(12):1717–23.
Haug AR, Rominger A, Mustafa M, Auernhammer C, Göke B, Schmidt GP, et al. Treatment with octreotide does not reduce tumor uptake of (68)Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med. 2011;52(11):1679–83.
Jahn U, Ilan E, Velikyan I, Fröss-Baron K, Lubberink M, Sundin A. Receptor depletion and recovery in small-intestinal neuroendocrine tumors and normal tissues after administration of a single intravenous dose of octreotide measured by (68)Ga-DOTATOC PET/CT. EJNMMI Res. 2021;11(1):118.
Ayati N, Lee ST, Zakavi R, Pathmaraj K, Al-Qatawna L, Poon A, et al. Long-acting somatostatin analog therapy differentially alters (68)Ga-DOTATATE uptake in normal tissues compared with primary tumors and metastatic lesions. J Nucl Med. 2018;59(2):223–7.
Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA. Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (Elect): a randomized, double-blind, placebo-controlled trial. Endocr Pract. 2016;22(9):1068–80.
de Vries-Huizing DMV, Versleijen MWJ, Sinaasappel M, Walraven I, Geluk-Jonker MM, Tesselaar MET, et al. Haematotoxicity during peptide receptor radionuclide therapy: baseline parameters differences and effect on patient’s therapy course. PLoS ONE. 2021;16(11): e0260073.
van Andel L, Aalbersberg EA, Geluk-Jonker MM, Stokkel MPM, Beijnen JH, Hendrikx JJMA. The development and validation of a high performance liquid chromatography method to determine the radiochemical purity of [177Lu]Lu-HA-DOTA-TATE in pharmaceutical preparations. J Chromatogr B. 2021;1171: 122605.
Sandström M, Ilan E, Karlberg A, Johansson S, Freedman N, Garske-Román U. Method dependence, observer variability and kidney volumes in radiation dosimetry of 177Lu-DOTATATE therapy in patients with neuroendocrine tumours. EJNMMI Physics. 2015;2(1):24.
Sandström M, Garske U, Granberg D, Sundin A, Lundqvist H. Individualized dosimetry in patients undergoing therapy with (177)Lu-DOTA-D-Phe (1)-Tyr (3)-octreotate. Eur J Nucl Med Mol Imaging. 2010;37(2):212–25.
Huizing DMV, Sinaasappel M, Dekker MC, Stokkel MPM, de Wit-van der Veen BJ. (177) Lutetium SPECT/CT: evaluation of collimator, photopeak and scatter correction. J Appl Clin Med Phys. 2020;21(9):272–7.
Peters SMB, Meyer Viol SL, van der Werf NR, de Jong N, van Velden FHP, Meeuwis A, et al. Variability in lutetium-177 SPECT quantification between different state-of-the-art SPECT/CT systems. EJNMMI Phys. 2020;7(1):9.
Ezziddin S, Reichmann K, Yong-Hing C, Damm M, Risse J, Ahmadzadehfar H, et al. Early prediction of tumour response to PRRT. The sequential change of tumour-absorbed doses during treatment with 177Lu-octreotate. Nuklearmedizin. 2013;52(5):170–7.
Garkavij M, Nickel M, Sjögreen-Gleisner K, Ljungberg M, Ohlsson T, Wingårdh K, et al. 177Lu-[DOTA0, Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer. 2010;116(S4):1084–92.
Common Terminology Criteria for Adverse Events v5.0. [Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.] Accessed on Nov 2, 2022.
Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J. 2005;52(5):605–11.
Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28(7):836–46.
Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2016;43(3):453–63.
Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42(1):5–19.
Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0), Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging. 2009;36(7):1138–46.
Hindorf C, Glatting G, Chiesa C, Lindén O, Flux G. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging. 2010;37(6):1238–50.
Garske-Román U, Sandström M, Fröss Baron K, Lundin L, Hellman P, Welin S, et al. Prospective observational study of (177)Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45(6):970–88.
Svensson J, Rydén T, Hagmarker L, Hemmingsson J, Wängberg B, Bernhardt P. A novel planar image-based method for bone marrow dosimetry in (177)Lu-DOTATATE treatment correlates with haematological toxicity. EJNMMI Phys. 2016;3(1):21.
Samenvatting van de productkenmerken Somatuline Autogel [Available from: https://www.geneesmiddeleninformatiebank.nl/smpc/h26301_smpc.pdf]. Accessed 1 Aug 2022.
Summary of product characteristics, labelling and package leaflet Sandostatin LAR [Available from: https://www.ema.europa.eu/en/documents/referral/sandostatin-lar-article-30-referral-annex-iii_en.pdf]. Acessed 1 Aug 2022.
Chicheportiche A, Ben-Haim S, Grozinsky-Glasberg S, Oleinikov K, Meirovitz A, Gross DJ, et al. Dosimetry after peptide receptor radionuclide therapy: impact of reduced number of post-treatment studies on absorbed dose calculation and on patient management. EJNMMI Phys. 2020;7(1):5.
Willowson KP, Eslick E, Ryu H, Poon A, Bernard EJ, Bailey DL. Feasibility and accuracy of single time point imaging for renal dosimetry following (177)Lu-DOTATATE (‘Lutate’) therapy. EJNMMI Phys. 2018;5(1):33.
Hou X, Brosch J, Uribe C, Desy A, Böning G, Beauregard J-M, et al. Feasibility of single-time-point dosimetry for radiopharmaceutical therapies. J Nucl Med. 2021;62(7):1006–11.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Chayenne Veerman, Hinke Siebinga, and Else Aalbersberg. The first draft of the manuscript was written by Chayenne Veerman and Else Aalbersberg and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was approved by the Institutional Research Board (IRBd21-187, approved July 26, 2021].
Consent to participate
All patients signed a general consent for the use of their data in retrospective studies.
Consent for publication.
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—General.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Veerman, C.H.A.M., Siebinga, H., de Vries-Huizing, D.M.V. et al. The effect of long-acting somatostatin analogues on the uptake of [177Lu]Lu-HA-DOTATATE. Eur J Nucl Med Mol Imaging 50, 1434–1441 (2023). https://doi.org/10.1007/s00259-022-06094-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06094-z