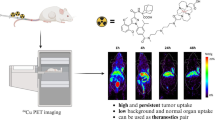
Abstract
Purpose
Despite its limitations, [123I]MIBG scintigraphy has been the standard for human norepinephrine transporter (hNET) imaging for several decades. Recently, [18F]MFBG has emerged as a promising PET alternative. This prospective trial aimed to evaluate safety, biodistribution, tumour lesion pharmacokinetics, and lesion targeting of [18F]MFBG and perform a head-to-head comparison with [123I]MIBG in neural crest tumour patients.
Methods
Six neural crest tumour patients (4 phaeochromocytoma, 1 paraganglioma, 1 neuroblastoma) with a recent routine clinical [123I]MIBG scintigraphy (interval: − 37–75 days) were included. Adult patients (n = 5) underwent a 30-min dynamic PET, followed by 3 whole-body PET/CT scans at 60, 120, and 180 min after injection of 4 MBq/kg [18F]MFBG. One minor participant underwent a single whole-body PET/CT at 60 min after administration of 2 MBq/kg [18F]MFBG. Normal organ uptake (SUVmean) and lesion uptake (SUVmax; tumour-to-background ratio (TBR)) were measured. Regional distribution volumes (VT) were estimated using a Logan graphical analysis in up to 6 lesions per patient. A lesion-by-lesion analysis was performed to compare detection ratios (DR), i.e. fraction of detected lesions, between [18F]MFBG and [123I]MIBG.
Results
[18F]MFBG was safe and well tolerated. Its biodistribution was overall similar to that of [123I]MIBG, with prominent uptake in the salivary glands, liver, left ventricle wall and adrenals, and mainly urinary excretion. In the phaeochromocytoma subgroup, the median VT was 37.4 mL/cm3 (range: 18.0–144.8) with an excellent correlation between VT and SUVmean at all 3 time points (R2: 0.92–0.94). Mean lesion SUVmax and TBR at 1 h after injection were 19.3 ± 10.7 and 23.6 ± 8.4, respectively. All lesions detected with [123I]MIBG were also observed with [18F]MFBG. The mean DR with [123I]MIBG was significantly lower than with [18F]MFBG (61.0% ± 26.7% vs. 99.8% ± 0.5% at 1 h; p = 0.043).
Conclusion
[18F]MFBG is a promising hNET imaging agent with favourable imaging characteristics and improved lesion targeting compared with [123I]MIBG scintigraphy.
Trial registration
Clinicaltrials.gov: NCT04258592 (Registered: 06 February 2020), EudraCT: 2019-003872-37A.




Similar content being viewed by others

Data availability
The datasets generated and/or analysed during the current study are not publicly available which is not approved by the Ethics Committee at UZ/KU Leuven due to patient’s confidentiality issues, but are available from the corresponding author on reasonable request.
References
Maguire LH, Thomas AR, Goldstein AM. Tumors of the neural crest: common themes in development and cancer. Dev Dyn. 2015;244:311–22.
Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11.
Pauwels E, Van Aerde M, Bormans G, Deroose CM. Molecular imaging of norepinephrine transporter-expressing tumors: current status and future prospects. Q J Nucl Med Mol Imaging. 2020;64:234–49.
Pandit-Taskar N, Modak S. Norepinephrine transporter as a target for imaging and therapy. J Nucl Med. 2017;58:39S–53S.
Bar-Sever Z, Biassoni L, Shulkin B, Kong G, Hofman MS, Lopci E, et al. Guidelines on nuclear medicine imaging in neuroblastoma. Eur J Nucl Med Mol Imaging. 2018;45:2009–24.
Taieb D, Hicks RJ, Hindie E, Guillet BA, Avram A, Ghedini P, et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging procedure standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46:2112–37.
Bombardieri E, Giammarile F, Aktolun C, Baum RP, Bischof Delaloye A, Maffioli L, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37:2436–46.
Pandit-Taskar N, Zanzonico P, Staton KD, Carrasquillo JA, Reidy-Lagunes D, Lyashchenko S, et al. Biodistribution and dosimetry of 18F-meta-fluorobenzylguanidine: a first-in-human PET/CT imaging study of patients with neuroendocrine malignancies. J Nucl Med. 2018;59:147–53.
Rotstein BH, Wang L, Liu RY, Patteson J, Kwan EE, Vasdev N, et al. Mechanistic studies and radiofluorination of structurally diverse pharmaceuticals with spirocyclic iodonium(III) ylides. Chem Sci. 2016;7:4407–17.
Celen S, Rokka J, Gilbert TM, Koole M, Vermeulen I, Serdons K, et al. Translation of HDAC6 PET imaging using [18F]EKZ-001-cGMP production and measurement of HDAC6 target occupancy in nonhuman primates. ACS Chem Neurosci. 2020;11:1093–101.
Baete K, Sevenois M, Pauwels E, Deroose CM. Verification of xSPECT quant for quantitative I-123-mIBG SPECT/CT imaging. Eur J Nucl Med Mol Imaging. 2020;47:S17–S8.
Grkovski M, Zanzonico PB, Modak S, Humm JL, Narula J, Pandit-Taskar N. F-18 meta-fluorobenzylguanidine PET imaging of myocardial sympathetic innervation. J Nucl Cardiol. 2022.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7.
Lewington V, Lambert B, Poetschger U, Sever ZB, Giammarile F, McEwan AJB, et al. 123I-mIBG scintigraphy in neuroblastoma: development of a SIOPEN semi-quantitative reporting, method by an international panel. Eur J Nucl Med Mol Imaging. 2017;44:234–41.
Garg PK, Garg S, Zalutsky MR. Synthesis and preliminary evaluation of Para- and meta-[18F]fluorobenzylguanidine. Nucl Med Biol. 1994;21:97–103.
Pauwels E, Celen S, Vandamme M, Leysen W, Baete K, Bechter O, et al. Improved resolution and sensitivity of [18F]MFBG PET compared with [123I]MIBG SPECT in a patient with a norepinephrine transporter-expressing tumour. Eur J Nucl Med Mol Imaging. 2021;48:313–5.
Jacobsson H, Hellstrom PM, Kogner P, Larsson SA. Different concentrations of I-123 MIBG and in-111 pentetreotide in the two main liver lobes in children: persisting regional functional differences after birth? Clin Nucl Med. 2007;32:24–8.
Jacobsson H, Johansson L, Kimiaei S, Larsson SA. Concentration of 123I-metaiodobenzylguanidine in left and right liver lobes. Findings indicate regional differences in function in the normal liver. Acta Radiol. 1999;40:224–8.
Moreau A, Giraudet AL, Kryza D, Borson-Chazot F, Bournaud C, Mognetti T, et al. Quantitative analysis of normal and pathologic adrenal glands with 18F-FDOPA PET/CT: focus on pheochromocytomas. Nucl Med Commun. 2017;38:771–9.
Noordzij W, Glaudemans A, Schaafsma M, van der Horst-Schrivers ANA, Slart R, van Beek AP, et al. Adrenal tracer uptake by 18F-FDOPA PET/CT in patients with pheochromocytoma and controls. Eur J Nucl Med Mol Imaging. 2019;46:1560–6.
Streby KA, Shah N, Ranalli MA, Kunkler A, Cripe TP. Nothing but NET: a review of norepinephrine transporter expression and efficacy of 131I-mIBG therapy. Pediatr Blood Cancer. 2015;62:5–11.
Wu J, Lin SF, Gallezot JD, Chan C, Prasad R, Thorn SL, et al. Quantitative analysis of dynamic 123I-mIBG SPECT imaging data in healthy humans with a population-based metabolite correction method. J Nucl Med. 2016;57:1226–32.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Deroose CM, Hindie E, Kebebew E, Goichot B, Pacak K, Taieb D, et al. Molecular imaging of gastroenteropancreatic neuroendocrine tumors: current status and future directions. J Nucl Med. 2016;57:1949–56.
Acknowledgements
The authors want to thank Mr. Kwinten Porters, Mr. Jef Van Loock, Mr. Wies Deckers, Mr. Matthijs Sevenois, Dr. Mathilde Vandamme, Mr. William Leysen, and the PET radiopharmacy team UZ Leuven for their skilled contributions. This research was funded by the project from “Kom op tegen Kanker”: “PET/MR imaging of the norepinephrine transporter and somatostatin receptor in neural crest and neuroendocrine tumours for better radionuclide therapy selection”. Christophe M. Deroose is a Senior Clinical Investigator from the Research Foundation – Flanders (FWO).
Funding
This research was funded by the project from “Kom op tegen Kanker”: “PET/MR imaging of the norepinephrine transporter and somatostatin receptor in neural crest and neuroendocrine tumours for better radionuclide therapy selection”. Christophe M. Deroose is a senior clinical investigator from the Research Foundation – Flanders (FWO).
Author information
Authors and Affiliations
Contributions
The study was designed by EP and CMD. Data collection, including patient-related activities, was done by EP, SC, KB, OB, MB, MR, PMC, SJ, KS, KVL, GB, and CMD. Data analysis and statistics were performed by EP, SC, MK, and CMD. The first draft was written by EP, and all authors revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approval was granted by the Ethics Committee Research UZ/KU Leuven (28 January 2020/S63142).
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1a and 3.
Competing interests
Paul M. Clement received study budget funds from AstraZeneca and was an advisory board member for AbbVie, AstraZeneca, Bayer, BMS, Daiichi-Sankyo, Leo Pharma, Merck Serono, MSD, Rakuten, Takeda, and Vifor Pharma, outside the scope of the submitted work. Christophe M. Deroose has been a consultant for Terumo, Ipsen, Sirtex, Bayer, and PSI CRO outside the scope of the submitted work. There are no other conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - General.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pauwels, E., Celen, S., Baete, K. et al. [18F] MFBG PET imaging: biodistribution, pharmacokinetics, and comparison with [123I] MIBG in neural crest tumour patients. Eur J Nucl Med Mol Imaging 50, 1134–1145 (2023). https://doi.org/10.1007/s00259-022-06046-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06046-7