Abstract
Background
Intraoperative molecular imaging (IMI) with folate-targeted NIR tracers has been shown to improve lesion localization in more than 80% of lung adenocarcinomas. However, mucinous adenocarcinomas (MAs) and invasive mucinous adenocarcinomas (IMAs) of the lung, which are variants of adenocarcinoma, appear to have decreased fluorescence despite appropriate folate receptor expression on the tumor surface. We hypothesized that the etiology may be related to light excitation and emission through non-Newtonian fluid (mucin) produced by goblet and columnar cancer cells.
Methods
Intraoperative data for 311 subjects were retrospectively reviewed from a prospectively collected 6-year database. For standardization, all patients underwent infusion of the same targeted molecular optical contrast agent (pafolacianine, folate receptor-targeted NIR fluorochrome) for lung cancer resections. Then, the ratio of the mean fluorescence intensity of the tumors and background tissues (TBR) was calculated. Tumors were examined for mucin, FRa, FRb, and immunofluorescent tracer uptake by a board-certified pathologist. The optical properties of mucin analyzed by imaging software were used to create in vitro gel models to explore the effects on NIR tracer fluorescence intensity.
Results
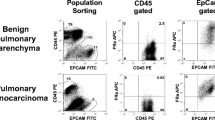
A large proportion (192, 62%) of the patients were female, with an average of 62.8 years and a 34-year mean pack smoking history. There were no severe (Clavien–Dindo > III) complications related to pafolacianine infusion. A total of 195 lesions in the study were adenocarcinomas, of which 19 (6.1%) were of the mucinous subtype. A total of 14/19 of the patients had a smoking history, and more than 74% of the IMA lesions were in the lower lobes. IMA lesions had a lower in situ TBR than nonmucinous adenocarcinomas (2.64 SD 0.23) vs (3.45 SD 0.11), respectively (p < 0.05). Only 9/19 (47%) were localized in situ. Tumor bisection and removal of mucin from IMAs significantly increased pafolacianine fluorescence, with resultant TBR not being significantly different from the control group (4.67 vs 4.89) (p = 0.19). Of the 16 lesions that underwent FR expression analysis, 15/16 had FR presence on cancer cells or tumor-associated macrophages in the tumor microenvironment. There was no statistically significant difference in fluorescence intensity during immunofluorescence analysis (4.99 vs 5.08) (p = 0.16). Physical removal of mucin from IMAs improved the TBR from 3.11 to 4.67 (p < 0.05). In vitro analysis of the impact of synthetic non-Newtonian fluid (agarose 0.5%) on NIR tracer fluorescence showed a decrease in MFI by a factor of 0.25 regardless of the concentration for each 5 mm thickness of mucin.
Conclusion
The mucinous subtype of lung adenocarcinomas presents a unique challenge in pafolacianine-targeted IMI-guided resections. The presence of non-Newtonian fluids presents a physical barrier that dampens the excitation of the tracer and fluorescence emission detected by the camera. Knowledge of this phenomenon can allow the surgeon to critically analyze lesion fluorescence parameters during IMI-guided lung cancer resections.







Similar content being viewed by others
Abbreviations
- IMI:
-
Intraoperative molecular imaging
- IMA:
-
Invasive mucinous adenocarcinoma
- FRa:
-
Folate receptor alpha
- FRb:
-
Folate receptor beta
- TBR:
-
Tumor to background ratio
- NIR:
-
Near infrared
References
Wood R, Taylor-Stokes G. Cost burden associated with advanced non-small cell lung cancer in Europe and influence of disease stage. BMC Cancer. 2019;19:214.
Xia W, et al. Improvement of survival for non-small cell lung cancer over time. Onco Targets Ther. 2017;10:4295–303.
Heuvers ME, Hegmans JP, Stricker BH, Aerts JG. Improving lung cancer survival; time to move on. BMC Pulm Med. 2012;12:77.
Surgery may improve survival rates for non-small cell lung cancer limited to the lung and surrounding affected glands. https://www.cochrane.org/CD004699/LUNGCA_surgery-may-improve-survival-rates-for-non-small-cell-lung-cancer-limited-to-the-lung-and-surrounding-affected-glandshttps://doi.org/10.1002/14651858.CD004699.pub2. Accessed 15 Jan 2022.
Azari F, Kennedy G, Singhal S. Intraoperative detection and assessment of lung nodules. Surg Oncol Clin. 2020;29:525–41.
Azari F, et al. Intraoperative molecular imaging clinical trials: a review of 2020 conference proceedings. JBO. 2021;26:050901.
Chi C, et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics. 2014;4:1072–84.
Gangadharan S, Sarkaria IN, Rice D, Murthy S, Braun J, Kucharczuk J, et al. Multi-institutional phase 2 clinical trial of intraoperative molecular imaging of lung cancer. Ann Thorac Surg. 2021;112(4):1150–9. https://doi.org/10.1016/j.athoracsur.2020.09.037.
O’Shannessy DJ, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–25.
De Jesus E, Keating JJ, Kularatne SA, Jiang J, Judy R, Predina J, et al. Comparison of folate receptor targeted optical contrast agents for intraoperative molecular imaging. Int J Mol Imaging. 2015;2015:469047. https://doi.org/10.1155/2015/469047.
Predina JD, et al. Identification of a folate receptor-targeted near-infrared molecular contrast agent to localize pulmonary adenocarcinomas. Mol Ther. 2018;26:390–403.
Newton AD, Predina JD, Nie S, Low PS, Singhal S. Intraoperative fluorescence imaging in thoracic surgery. J Surg Oncol. 2018;118:344–55.
Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017;6(5):508–512. https://doi.org/10.21037/tlcr.2017.06.10.
Travis WD, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60.
Goldstein NS, Thomas M. Mucinous and nonmucinous bronchioloalveolar adenocarcinomas have distinct staining patterns with thyroid transcription factor and cytokeratin 20 antibodies. Am J Clin Pathol. 2001;116:319–25.
Shim HS, et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2015;10:1156–62.
Yoshizawa A, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64.
Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86–100.
Chen S, et al. Understanding optical reflectance contrast for real-time characterization of epithelial precursor lesions. Bioeng Transl Med. 2019;4:e10137.
Comprehensive Biotechnology | ScienceDirect. https://www.sciencedirect.com/referencework/9780080885049/comprehensive-biotechnology. Accessed 21 Dec 2021.
Azari F, Kennedy G, Bernstein E, Delikatny J, Lee JYK, Kucharczuk J, et al. Evaluation of OTL38-generated tumor-to-background ratio in intraoperative molecular imaging-guided lung cancer resections. Mol Imaging Biol. 2021;1–12. https://doi.org/10.1007/s11307-021-01618-9.
Kennedy, G. T. et al. Targeted intraoperative molecular imaging for localizing nonpalpable tumors and quantifying resection margin distances. JAMA Surg (2021) https://doi.org/10.1001/jamasurg.2021.3757
Shi J, Li D, Liang D, He Y. Epidemiology and prognosis in young lung cancer patients aged under 45 years old in northern China. Sci Rep. 2021;11:6817.
Thomas A, Chen Y, Yu T, Jakopovic M, Giaccone G. Trends and characteristics of young non-small cell lung cancer patients in the United States. Front Oncol. 2015;5:113.
Sagerup CMT, Småstuen M, Johannesen TB, Helland Å, Brustugun OT. Sex-specific trends in lung cancer incidence and survival: a population study of 40 118 cases. Thorax. 2011;66:301–7.
Kennedy GT, et al. The optical biopsy: a novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Ann Surg. 2015;262:602–9.
Zargham M, Moradi A-R, Najafi A. Experimental investigation of surface tension in Newtonian and non-Newtonian fluids with optical diffractometry. In Martins Costa MFPC, editors. Proc. SPIE 8785, 8th Iberoamerican Optics Meeting and 11th Latin American Meeting on Optics, Lasers, and Applications, 8785DN (2013). https://doi.org/10.1117/12.2026373.
Eberhard U, et al. Mapping the local viscosity of non-Newtonian fluids flowing through disordered porous structures. Sci Rep. 2020;10:11733.
Cao, J. et al. Recent progress in NIR-II contrast agent for biological imaging. Frontiers in Bioengineering and Biotechnology 7, (2020)
Acknowledgements
Figure panels edited with BioRender.
Funding
Dr. Azari was supported by the training grant in Surgical Oncology by the National Institutes of Health (T32CA251063-01), the Society of Thoracic Surgeons Thoracic Surgery Foundation Research Award, and the Stephen CC Cheung Fellowship in Surgical Oncology. Dr. Singhal was supported by the National Institutes of Health (NIH P01CA254859) and the State of Pennsylvania Health Research Formula Fund. Dr. Kennedy was supported by the American Philosophical Society and the National Institutes of Health (grant F32 CA254210-01). Dr. Sullivan has an NIH T32 postdoctoral fellowship (NIH T32 CA009140).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and approved by the University of Pennsylvania Institutional Review Board. The study complies with the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Translational research.
Supplementary Information
Below is the link to the electronic supplementary material.
Video Demonstration of mucin removal and its impact on fluorescence emission intensity change during pafolacianine-guided lung cancer resection of invasive mucinous adenocarcinoma. (MP4 284648 KB)
Rights and permissions
About this article
Cite this article
Azari, F., Kennedy, G., Chang, A. et al. Presence of non-Newtonian fluid in invasive pulmonary mucinous adenocarcinomas impacts fluorescence during intraoperative molecular imaging of lung cancer. Eur J Nucl Med Mol Imaging 49, 4406–4418 (2022). https://doi.org/10.1007/s00259-022-05912-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05912-8