Abstract
Purpose
The aim of this [18F]-FDG PET study was to determine the diagnostic value of the cortex/striatum metabolic ratio in a large cohort of patients suffering from autoimmune encephalitis (AE) and to search for correlations with the course of the disease.
Methods
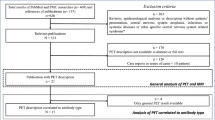
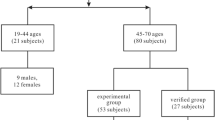
We retrospectively collected clinical and paraclinical data of patients with AE, including brain 18F-FDG PET/CT. Whole-brain statistical analysis was performed using SPM8 software after activity parametrization to the striatum in comparison to healthy subjects. The discriminative performance of this metabolic ratio was evaluated in patients with AE using receiver operating characteristic curves against 44 healthy subjects and a control group of 688 patients with MCI. Relationship between cortex/striatum metabolic ratios and clinical/paraclinical data was assessed using univariate and multivariate analysis in patients with AE.
Results
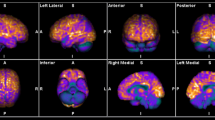
Fifty-six patients with AE were included. In comparison to healthy subjects, voxel-based statistical analysis identified one large cluster (p-cluster < 0.05, FWE corrected) of widespread decreased cortex/striatum ratio in patients with AE. The mean metabolic ratio was significantly lower for AE patients (1.16 ± 0.13) than that for healthy subjects (1.39 ± 0.08; p < 0.001) and than that for MCI patients (1.32 ± 0.11; p < 0.001). A ratio threshold of 1.23 allowed to detect AE patients with a sensitivity of 71% and a specificity of 82% against MCI patients, and 98% against healthy subjects. A lower cortex/striatum metabolic ratio had a trend towards shorter delay before 18F-FDG PET/CT (p = 0.07) in multivariate analysis.
Conclusion
The decrease in the cortex/striatal metabolic ratio has a good early diagnostic performance for the differentiation of AE patients from controls.



Similar content being viewed by others
Data availability
The PET data that support the findings are available from the corresponding author upon reasonable request.
Code availability
Not applicable
References
Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378:840–51.
Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65.
Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019;92:e244–52.
Hébert J, Day GS, Steriade C, Wennberg RA, Tang-Wai DF. Long-term cognitive outcomes in patients with autoimmune encephalitis. Can J Neurol Sci. 2018;45:540–4.
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404.
Ducray F, Demarquay G, Graus F, Decullier E, Antoine J-C, Giometto B, et al. Seronegative paraneoplastic cerebellar degeneration: the PNS Euronetwork experience. Eur J Neurol. 2014;21:731–5.
Hébert J, Gros P, Lapointe S, Amtashar FS, Steriade C, Maurice C, et al. Searching for autoimmune encephalitis: beware of normal CSF. J Neuroimmunol. 2020;345:577285.
Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83:166–77.
Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123(Pt 7):1481–94.
Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77.
Morbelli S, Djekidel M, Hesse S, Pagani M, Barthel H, Neuroimaging Committee of the European Association of Nuclear Medicine (EANM), et al. Role of (18)F-FDG-PET imaging in the diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:1009–10.
Sheikhbahaei S, Marcus CV, Fragomeni RS, Rowe SP, Javadi MS, Solnes LB. Whole-body 18F-FDG PET and 18F-FDG PET/CT in patients with suspected paraneoplastic syndrome: a systematic review and meta-analysis of diagnostic accuracy. J Nucl Med. 2017;58:1031–6.
Wagner JN, Kalev O, Sonnberger M, Krehan I, von Oertzen TJ. Evaluation of clinical and paraclinical findings for the differential diagnosis of autoimmune and infectious encephalitis. Front Neurol. 2018;9:434.
Li L, Sun L, Du R, Zheng Y, Dai F, Ma Q, et al. Application of the 2016 diagnostic approach for autoimmune encephalitis from Lancet Neurology to Chinese patients. BMC Neurol. 2017;17:195.
Bordonne M, Chawki MB, Doyen M, Kas A, Guedj E, Tyvaert L et al (2021) Brain 18F-FDG PET for the diagnosis of autoimmune encephalitis: a systematic review and a meta-analysis. Bordonne M, Chawki MB, Doyen M, Kas A, Guedj E, Tyvaert L, Verger A.Eur J Nucl Med Mol Imaging. 2021 Mar 7. https://doi.org/10.1007/s00259-021-05299-y
Morbelli S, Arbizu J, Booij J, Chen M-K, Chetelat G, Cross DJ, et al. The need of standardization and of large clinical studies in an emerging indication of [18F]FDG PET: the autoimmune encephalitis. Eur J Nucl Med Mol Imaging. 2017;44:353–7.
Cistaro A, Caobelli F, Quartuccio N, Fania P, Pagani M. Uncommon 18F-FDG-PET/CT findings in patients affected by limbic encephalitis: hyper-hypometabolic pattern with double antibody positivity and migrating foci of hypermetabolism. Clin Imaging. 2015;39:329–33.
Turpin S, Martineau P, Levasseur M-A, Meijer I, Décarie J-C, Barsalou J, et al. 18F-Flurodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) findings in children with encephalitis and comparison to conventional imaging. Eur J Nucl Med Mol Imaging. 2019;46:1309–24.
Fisher RE, Patel NR, Lai EC, Schulz PE. Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med. 2012;37:e213-218.
Quartuccio N, Caobelli F, Evangelista L, Alongi P, Kirienko M, De Biasi V, et al. The role of PET/CT in the evaluation of patients affected by limbic encephalitis: a systematic review of the literature. J Neuroimmunol. 2015;284:44–8.
Probasco JC, Solnes L, Nalluri A, Cohen J, Jones KM, Zan E, et al. Decreased occipital lobe metabolism by FDG-PET/CT: an anti-NMDA receptor encephalitis biomarker. Neurol Neuroimmunol Neuroinflamm. 2018;5:e413.
Wei Y-C, Tseng J-R, Wu C-L, Su F-C, Weng W-C, Hsu C-C, et al. Different FDG-PET metabolic patterns of anti-AMPAR and anti-NMDAR encephalitis: case report and literature review. Brain Behav. 2020;10:e01540.
Rey C, Koric L, Guedj E, Felician O, Kaphan E, Boucraut J, et al. Striatal hypermetabolism in limbic encephalitis. J Neurol. 2012;259:1106–10.
Probasco JC, Solnes L, Nalluri A, Cohen J, Jones KM, Zan E, et al. Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e352.
Castagnoli H, Manni C, Marchesani F, Rossi G, Fattori S, Capoccetti F. The role of 18F-FDG PET/CT in management of paraneoplastic limbic encephalitis combined with small cell lung cancer: A case report. Medicine (Baltimore). 2019;98:e16593.
Cózar Santiago MDP, Sanchez Jurado R, Sanz Llorens R, Aguilar Barrios JE, Ferrer RJ. Limbic encephalitis diagnosed with 18F-FDG PET/CT. Clin Nucl Med. 2016;41:e101-103.
Maffione AM, Chondrogiannis S, Ferretti A, Al-Nahhas A, Rubello D. Correlative imaging with (18)F-FDG PET/CT and MRI in paraneoplastic limbic encephalitis. Clin Nucl Med. 2013;38:463–4.
Moloney P, Boylan R, Elamin M, O’Riordan S, Killeen R, McGuigan C. Semi-quantitative analysis of cerebral FDG-PET reveals striatal hypermetabolism and normal cortical metabolism in a case of VGKCC limbic encephalitis. Neuroradiol J. 2017;30:160–3.
Moubtakir A, Dejust S, Godard F, Messaoud L, Morland D. 18F-FDG PET/CT in anti-NMDA receptor encephalitis: typical pattern and follow-up. Clin Nucl Med. 2018;43:520–1.
Scheid R, Lincke T, Voltz R, von Cramon DY, Sabri O. Serial 18F-fluoro-2-deoxy-D-glucose positron emission tomography and magnetic resonance imaging of paraneoplastic limbic encephalitis. Arch Neurol. 2004;61:1785–9.
Gaeta MC, Godani M, Nunziata R, Capellini C, Ciarmiello A. Early detection of encephalitis with (18)F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2015;34:188–90.
Liu X, Shan W, Zhao X, Ren J, Ren G, Chen C, et al. The clinical value of 18 F-FDG-PET in autoimmune encephalitis associated with LGI1 antibody. Front Neurol. 2020;11:418.
Dodich A, Cerami C, Iannaccone S, Marcone A, Alongi P, Crespi C, et al. Neuropsychological and FDG-PET profiles in VGKC autoimmune limbic encephalitis. Brain Cogn. 2016;108:81–7.
Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T, et al. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83:681–6.
Wegner F, Wilke F, Raab P, Tayeb SB, Boeck A-L, Haense C, et al. Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-D-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol. 2014;14:136.
Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260:2744–53.
Lee BY, Newberg AB, Liebeskind DS, Kung J, Alavi A. FDG-PET findings in patients with suspected encephalitis. Clin Nucl Med. 2004;29:620–5.
Masangkay N, Basu S, Moghbel M, Kwee T, Alavi A. Brain 18F-FDG-PET characteristics in patients with paraneoplastic neurological syndrome and its correlation with clinical and MRI findings. Nucl Med Commun. 2014;35:1038–46.
Moreno-Ajona D, Prieto E, Grisanti F, Esparragosa I, Sánchez Orduz L, Gállego Pérez-Larraya J et al 18F-FDG-PET imaging patterns in autoimmune encephalitis: impact of image analysis on the results. Diagnostics (Basel). 2020;10(6):356. https://doi.org/10.3390/diagnostics10060356.
Novy J, Allenbach G, Bien CG, Guedj E, Prior JO, Rossetti AO. FDG-PET hyperactivity pattern in anti-NMDAr encephalitis. J Neuroimmunol. 2016;297:156–8.
Yakushev I, Landvogt C, Buchholz H-G, Fellgiebel A, Hammers A, Scheurich A, et al. Choice of reference area in studies of Alzheimer’s disease using positron emission tomography with fluorodeoxyglucose-F18. Psychiatry Res. 2008;164:143–53.
Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz H-G, et al. SPM-based count normalization provides excellent discrimination of mild Alzheimer’s disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009;44:43–50.
Nugent S, Croteau E, Potvin O, Castellano C-A, Dieumegarde L, Cunnane SC, et al. Selection of the optimal intensity normalization region for FDG-PET studies of normal aging and Alzheimer’s disease. Sci Rep. 2020;10:9261.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A, et al. Diagnostic value of 18F-FDG PET/CT versus MRI in the setting of antibody-specific autoimmune encephalitis. J Nucl Med. 2017;58:1307–13.
Lv R-J, Pan J, Zhou G, Wang Q, Shao X-Q, Zhao X-B, et al. Semi-quantitative FDG-PET analysis increases the sensitivity compared with visual analysis in the diagnosis of autoimmune encephalitis. Front Neurol. 2019;10:576.
Tripathi M, Tripathi M, Roy SG, Parida GK, Ihtisham K, Dash D, et al. Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology. 2018;60:189–98.
Funding
The local PET database of healthy controls was funded by APHM (regional PHRC; NCT00484523).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics approval
The retrospective observations required no ethical approval requirement other than informed consent and non-opposition (MR-004 French type). The local PET database of healthy controls was acquired in accordance with the Declaration of Helsinki, with informed written consent from the patients and approval from the “CPP Sud Méditerranée V” ethics committee.
Consent to participate
Informed written consent was obtained from all individual participants included in the study.
Consent for publication
Informed written consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
De Leiris, N., Ruel, B., Vervandier, J. et al. Decrease in the cortex/striatum metabolic ratio on [18F]-FDG PET: a biomarker of autoimmune encephalitis. Eur J Nucl Med Mol Imaging 49, 921–931 (2022). https://doi.org/10.1007/s00259-021-05507-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05507-9