Abstract
Purpose
Phosphodiesterase 10A (PDE10A) is a dual substrate enzyme highly enriched in dopamine-receptive striatal medium spiny neurons, which are involved in psychiatric disorders such as alcohol use disorders (AUD). Although preclinical studies suggest a correlation of PDE10A mRNA expression in neuronal and behavioral responses to alcohol intake, little is known about the effects of alcohol exposure on in vivo PDE10A activity in relation to apparent risk factors for AUD such as decision-making and anxiety.
Methods
We performed a longitudinal [18F]JNJ42259152 microPET study to evaluate PDE10A changes over a 9-week intermittent access to alcohol model, including 6 weeks of alcohol exposure, 2 weeks of abstinence followed by 1 week relapse. Parametric PDE10A-binding potential (BPND) images were generated using a Logan reference tissue model with cerebellum as reference region and were analyzed using both a volume-of-interest and voxel-based approach. Moreover, individual decision-making and anxiety levels were assessed with the rat Iowa Gambling Task and open-field test over the IAE model.
Results
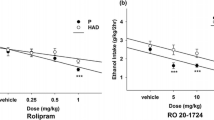
We observed an increased alcohol preference especially in those animals that exhibited poor initial decision-making. The first 2 weeks of alcohol exposure resulted in an increased striatal PDE10A binding (> 10%). Comparing PDE10A-binding potential after 2 versus 4 weeks of exposure showed a significant decreased PDE10A in the caudate-putamen and nucleus accumbens (pFWE-corrected < 0.05). This striatal PDE10A decrease was related to alcohol consumption and preference. Normalization of striatal PDE10A to initial levels was observed after 1 week of relapse, apart from the globus pallidus.
Conclusion
This study shows that chronic voluntary alcohol consumption induces a reversible increased PDE10A enzymatic availability in the striatum, which is related to the amount of alcohol preference. Thus, PDE10A-mediated signaling plays an important role in modulating the reinforcing effects of alcohol, and the data suggest that PDE10A inhibition may have beneficial behavioral effects on alcohol intake.





Similar content being viewed by others
Data availability of data and materials
Please contact the authors for data request.
Change history
14 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00259-022-05802-z
Abbreviations
- AUD:
-
Alcohol use disorders
- BP:
-
Binding potential
- cAMP:
-
Cyclic adenosine monophosphate
- cGMP:
-
Cyclic guanosine monophosphate
- D1:
-
Dopamine receptor type 1
- D2:
-
Dopamine receptor type 2
- IAE:
-
Intermittent access to ethanol
- MSNs:
-
Medium spiny neurons
- NuAc:
-
Nucleus accumbens
- OFT:
-
Open-field test
- PDE:
-
Phosphodiesterase
- PDE10A:
-
Phosphodiesterase 10A
- rIGT:
-
Rat Iowa Gambling Task
- VOI:
-
Volumes of interest
References
Leurquin-Sterk G, Ceccarini J, Crunelle CL, Weerasekera A, de Laat B, Himmelreich U, et al. Cerebral dopaminergic and glutamatergic transmission relate to different subjective responses of acute alcohol intake: an in vivo multimodal imaging study. Addict Biol. 2018;23:931–44.
de Laat B, Weerasekera A, Leurquin-Sterk G, Gsell W, Bormans G, Himmelreich U, et al. Effects of alcohol exposure on the glutamatergic system: a combined longitudinal 18 F-FPEB and 1 H-MRS study in rats. Addic Biol. 2019;24:696–706.
Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of μ-opioid receptors. Neuroscience. 2008;153:240–8.
Logrip ML. Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol. 2015;49:795–802.
Sanderson TM, Sher E. The role of phosphodiesterases in hippocampal synaptic plasticity. Neuropharmacology. 2013;74:86–95.
García-Barroso C, Ugarte A, Martínez M, Rico AJ, Lanciego JL, Franco R, et al. Phosphodiesterase inhibition in cognitive decline. J Alzheimers Dis. 2014;42:S561–73.
Peng S, Sun H, Zhang X, Liu G, Wang G. Effects of selective phosphodiesterases-4 inhibitors on learning and memory: a review of recent research. Cell Biochem Biophys. 2014;70:83–5.
Kranz K, Warnecke A, Lenarz T, Durisin M, Scheper V. Phosphodiesterase type 4 Inhibitor rolipram improves survival of spiral ganglion neurons in vitro. Sokolowski B, editor. PLoS One. 2014;9:e92157.
Wilson L, Brandon N. Emerging Biology of PDE10A. Curr Pharm Des. 2014;21:378–88.
Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, et al. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 2003;985:113–26.
Jäger R, Russwurm C, Schwede F, Genieser H-G, Koesling D, Russwurm M. Activation of PDE10 and PDE11 phosphodiesterases. The Journal of biological chemistry. J Biol Chem. 2012;287:1210–9.
Gross-Langenhoff M, Hofbauer K, Weber J, Schultz A, Schultz JE. cAMP is a ligand for the tandem GAF domain of human phosphodiesterase 10 and cGMP for the tandem GAF domain of phosphodiesterase 11. The Journal of biological chemistry. J Biol Chem. 2006;281:2841–6.
Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–71.
Dlaboga D, Hajjhussein H, O’Donnell JM. Chronic haloperidol and clozapine produce different patterns of effects on phosphodiesterase-1B, -4B, and -10A expression in rat striatum. Neuropharmacol. 2008;54:745–54.
Giorgi M, Melchiorri G, Nuccetelli V, D’Angelo V, Martorana A, Sorge R, et al. PDE10A and PDE10A-dependent cAMP catabolism are dysregulated oppositely in striatum and nucleus accumbens after lesion of midbrain dopamine neurons in rat: a key step in parkinsonism physiopathology. Neurobiol Dis. 2011;43:293–303.
Ooms M, Celen S, De Hoogt R, Lenaerts I, Liebregts J, Vanhoof G, et al. Striatal phosphodiesterase 10A availability is altered secondary to chronic changes in dopamine neurotransmission. EJNMMI Radiopharm Chem. 2017;1:3.
Niccolini F, Foltynie T, Reis Marques T, Muhlert N, Tziortzi AC, Searle GE, et al. Loss of phosphodiesterase 10A expression is associated with progression and severity in Parkinson’s disease. Brain. 2015;138:3003–15.
Pagano G, Niccolini F, Wilson H, Yousaf T, Khan NL, Martino D, et al. Comparison of phosphodiesterase 10A and dopamine transporter levels as markers of disease burden in early Parkinson’s disease. Movement Disord. 2019;34:1505–15.
Beaumont V, Zhong S, Lin H, Xu W, Bradaia A, Steidl E, et al. Phosphodiesterase 10A inhibition improves cortico-basal ganglia function in Huntington’s disease models. Neuron. 2016;92:1220–37.
Wilson H, Niccolini F, Haider S, Marques TR, Pagano G, Coello C, et al. Loss of extra-striatal phosphodiesterase 10A expression in early premanifest Huntington’s disease gene carriers. J Neurol Sci. 2016;368:243–8.
Koole M, Van Laere K, Ahmad R, Ceccarini J, Bormans G, Vandenberghe W. Brain PET imaging of phosphodiesterase 10A in progressive supranuclear palsy and Parkinson’s disease. Movement Disord. 2017;32:943–5.
Ahmad R, Bourgeois S, Postnov A, Schmidt ME, Bormans G, Van Laere K, et al. PET imaging shows loss of striatal PDE10A in patients with Huntington disease. Neurology. 2014;82:279–81.
Persson J, Szalisznyó K, Antoni G, Wall A, Fällmar D, Zora H, et al. Phosphodiesterase 10A levels are related to striatal function in schizophrenia: a combined positron emission tomography and functional magnetic resonance imaging study. Eur Arch Psychiatry Clin Neurosci. 2020;270:451–9.
Bodén R, Persson J, Wall A, Lubberink M, Ekselius L, Larsson E-M, et al. Striatal phosphodiesterase 10A and medial prefrontal cortical thickness in patients with schizophrenia: a PET and MRI study. Transl Psychiatry. 2017;7:e1050.
Chappie T, Humphrey J, Menniti F, Schmidt C. PDE10A inhibitors: an assessment of the current CNS drug discovery landscape. Curr Opin Drug Discov Devel. 2009;12:458–67.
Mu Y, Ren Z, Jia J, Gao B, Zheng L, Wang G, et al. Inhibition of phosphodiesterase10A attenuates morphine-induced conditioned place preference. Mol Brain. 2014;7:70.
Liddie S, Anderson KL, Paz A, Itzhak Y. The effect of phosphodiesterase inhibitors on the extinction of cocaine-induced conditioned place preference in mice. J Psychopharmacol. 2012;26:1375–82.
Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addict Biol. 2012;17:920–33.
Logrip ML, Zorrilla EP. Differential changes in amygdala and frontal cortex Pde10a expression during acute and protracted withdrawal. Front Integr Neurosci. 2014;8:30.
Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF, Zorrilla EP. Phosphodiesterase 10A regulates alcohol and saccharin self-administration in rats. Neuropsychopharmacology. 2014;39:1722–31.
Celen S, Koole M, Ooms M, De Angelis M, Sannen I, Cornelis J, et al. Preclinical evaluation of [18F]JNJ42259152 as a PET tracer for PDE10A. Neuroimage. 2013;82:13–22.
Logrip ML, Gainey SC. Sex differences in the long-term effects of past stress on alcohol self-administration, glucocorticoid sensitivity and phosphodiesterase 10A expression. Neuropharmacology. 2020;164:107857. https://doi.org/10.1016/j.neuropharm.2019.107857.
Hsu YT, Liao G, Bi X, Oka T, Tamura S, Baudry M. The PDE10A inhibitor, papaverine, differentially activates ERK in male and female rat striatal slices. Neuropharmacology. 2011;61:1275–81.
Fazio P, Schain M, Mrzljak L, Amini N, Nag S, Al-Tawil N, et al. Patterns of age related changes for phosphodiesterase type-10A in comparison with dopamine D2/3 receptors and sub-cortical volumes in the human basal ganglia: A PET study with 18F-MNI-659 and 11C-raclopride with correction for partial volume effect. Neuroimage. 2017;152:330–9.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23.
Laat B, Weerasekera A, Leurquin-Sterk G, Gsell W, Bormans G, Himmelreich U, et al. Effects of alcohol exposure on the glutamatergic system: a combined longitudinal 18F-FPEB and 1H-MRS study in rats. Addict Biol. 2019;24:696–706.
Kimbrough A, Kim S, Cole M, Brennan M, George O. Intermittent access to ethanol drinking facilitates the transition to excessive drinking after chronic intermittent ethanol vapor exposure. Alcohol Clin Exp Res. 2017;41:1502.
Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacol. 2009;34:2329–43.
Van Laere K, Ahmad RU, Hudyana H, Dubois K, Schmidt ME, Celen S, et al. Quantification of 18F-JNJ-42259152, a novel phosphodiesterase 10A PET tracer: Kinetic modeling and test-retest study in human brain. J Nucl Med. 2013;54:1285–93.
Ooms M, Attili B, Celen S, Koole M, Verbruggen A, Van Laere K, et al. [18F]JNJ42259152 binding to phosphodiesterase 10A, a key regulator of medium spiny neuron excitability, is altered in the presence of cyclic AMP. J Neurochem. 2016;139:897–906.
Celen S, Koole M, De Angelis M, Sannen I, Chitneni SK, Alcazar J, et al. Preclinical evaluation of 18F-JNJ41510417 as a radioligand for PET imaging of phosphodiesterase-10A in the brain. J Nucl Med. 2010;51:1584–91.
Casteels C, Vermaelen P, Nuyts J, Van Der Linden A, Baekelandt V, Mortelmans L, et al. Construction and evaluation of multitracer small-animal PET probabilistic atlases for voxel-based functional mapping of the rat brain. J Nucl Med. 2006;47:1858–66.
Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–52.
Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–4.
Tollefson S, Gertler J, Himes ML, Paris J, Kendro S, Lopresti B, et al. Imaging phosphodiesterase-10a availability in cocaine use disorder with [11 C]IMA107 and PET. Synapse. 2019;73:e22070.
Wen R-T, Zhang F-F, Zhang H-T. Cyclic nucleotide phosphodiesterases: potential therapeutic targets for alcohol use disorder. Psychopharmacology. 2018;235(6):1793–805. https://doi.org/10.1007/s00213-018-4895-7.
Piccart E, De Backer J-F, Gall D, Lambot L, Raes A, Vanhoof G, et al. Genetic deletion of PDE10A selectively impairs incentive salience attribution and decreases medium spiny neuron excitability. Behav Brain Res. 2014;268:48–54.
Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG. Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry. 2014;171:881–8.
Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6.
Martinez D, Gil R, Slifstein M, Hwang D-R, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiat. 2005;58:779–86.
Heinz A, Siessmeier T, Wrase J, Buchholz HG, Gründer G, Kumakura Y, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–20.
Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, et al. Preclinical characterization of selective phosphodiesterase 10a inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther. 2008;325:681–90.
Nawrocki AR, Rodriguez CG, Toolan DM, Price O, Henry M, Forrest G, et al. Genetic deletion and pharmacological inhibition of phosphodiesterase 10A protects mice from diet-induced obesity and insulin resistance. Diabetes. 2014;63:300–11.
Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism: Clin Exp Res. 2007;31:1505–15.
Grauer SM, Pulito VL, Navarra RL, Kelly MP, Kelley C, Graf R, et al. Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J Pharmacol Exp Ther. 2009;331:574–90.
Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izídio GS. Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res. 2008;193:277–88.
Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: Who is at a greater risk for development of alcoholic complication? Life Sci 2010;87(5–6):133–8. https://doi.org/10.1016/j.lfs.2010.06.002
Pohjalainen T, Rinne JO, Någren K, Syvälahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998;155:768–73.
Acknowledgements
The authors would like to thank Tinne Buelens and Ann Van Santvoort for their excellent technical assistance and the local radiopharmacy team for the tracer productions.
Funding
This work was funded by a research grant to JC from the Research Foundation Flanders (FWO/1508415 N). JC is a postdoctoral fellow from FWO (FWO/12R1619N). YEK is a SB PhD fellow at FWO (FWO/1S50320N), BdL received a PhD fellowship from the Flemish Agency for Innovation by Science and Technology, and KVL is senior clinical research fellow for the FWO. GS, MO, JMH, and GB have no competing financial interests to report in relation to this work.
Author information
Authors and Affiliations
Contributions
The experimental setup was designed by BdL and JC. BdL, GS and MO performed data collection. Data analysis was conducted by YEK and JC. The manuscript was written by YEK and JC, supported by BdL, GS, MO, JMH, GB and KVL. All authors revised the manuscript and accepted the final version.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were conducted according to the European Communities Council Directive of November 24, 1986 (86/609/EEC) and approved by the Animal Ethics Committees of the University of Leuven.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
Supplementary Information
ESM 1
(DOCX 469 kb)
Rights and permissions
About this article
Cite this article
de Laat, B., Kling, Y.E., Schroyen, G. et al. Effects of chronic voluntary alcohol consumption on PDE10A availability: a longitudinal behavioral and [18F]JNJ42259152 PET study in rats. Eur J Nucl Med Mol Imaging 49, 492–502 (2022). https://doi.org/10.1007/s00259-021-05448-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05448-3