Abstract
Purpose
Prostate-specific membrane antigen (PSMA), a type-II integral membrane protein highly expressed in prostate cancer, has been extensively used as a target for imaging and therapy. Among the available PET radiotracers, the low molecular weight agents that bind to PSMA are proving particularly effective. We present the dosimetry results for 18F-DCFPyL in nine patients with metastatic prostate cancer.
Methods
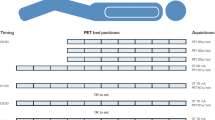
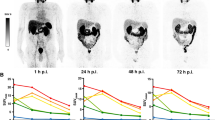
Nine patients were imaged using sequential PET/CT scans at approximately 1, 12, 35 and 70 min, and a final PET/CT scan at approximately 120 min after intravenous administration of 321 ± 8 MBq (8.7 ± 0.2 mCi) of18F-DCFPyL. Time-integrated-activity coefficients were calculated and used as input in OLINDA/EXM software to obtain dose estimates for the majority of the major organs. The absorbed doses (AD) to the eye lens and lacrimal glands were calculated using Monte-Carlo models based on idealized anatomy combined with patient-specific volumes and activity from the PET/CT scans. Monte-Carlo based models were also developed for calculation of the dose to two major salivary glands (parotid and submandibular) using CT-based patient-specific gland volumes.
Results
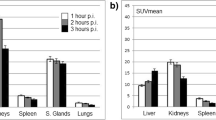
The highest calculated mean AD per unit administered activity of 18F was found in the lacrimal glands, followed by the submandibular glands, kidneys, urinary bladder wall, and parotid glands. The S-values for the lacrimal glands to the eye lens (0.42 mGy/MBq h), the tear film to the eye lens (1.78 mGy/MBq h) and the lacrimal gland self-dose (574.10 mGy/MBq h) were calculated. Average S-values for the salivary glands were 3.58 mGy/MBq h for the parotid self-dose and 6.78 mGy/MBq h for the submandibular self-dose. The resultant mean effective dose of 18F-DCFPyL was 0.017 ± 0.002 mSv/MBq.
Conclusions
18F-DCFPyL dosimetry in nine patients was obtained using novel models for the lacrimal and salivary glands, two organs with potentially dose-limiting uptake for therapy and diagnosis which lacked pre-existing models.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59.
Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol. 1999;36:31–5.
Nuñez R, Macapinlac HA, Yeung HW, et al. Combined 18F-FDG and 11C-methionine PET scans in patients with newly progressive metastatic prostate cancer. J Nucl Med. 2002;43:46–55.
Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996;199:751–6.
Rowe SP, Gorin MA, Allaf ME, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19:223–30.
Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28–53.
Lawrentschuk N, Davis ID, Bolton DM, Scott AM. Positron emission tomography and molecular imaging of the prostate: an update. BJU Int. 2006;97:923–31.
De Jong I, Pruim J, Elsinga P, Vaalburg W, Mensink H. 11C-choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol. 2003;44:32–9.
Picchio M, Messa C, Landoni C, et al. Value of [11C] choline-positron emission tomography for re-staging prostate cancer: a comparison with [18F] fluorodeoxyglucose-positron emission tomography. J Urol. 2003;169:1337–40.
Greco C, Cascini G, Tamburrini O. Is there a role for positron emission tomography imaging in the early evaluation of prostate cancer relapse? Prostate Cancer Prostatic Dis. 2008;11:121–8.
Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207.
Scattoni V, Picchio M, Suardi N, et al. Detection of lymph-node metastases with integrated [11 C] choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur Urol. 2007;52:423–9.
Schuster DM, Nanni C, Fanti S. PET tracers beyond FDG in prostate cancer. Semin Nucl Med. 2016;46:507–21.
Pandit-Taskar N, O’Donoghue JA, Durack JC, et al. A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res. 2015;21:5277–85.
Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–53.
Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–91.
Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–74.
Evangelista L, Briganti A, Fanti S, et al. New clinical indications for 18F/11C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol. 2016;70:161–75.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry – standardization of nomenclature. J Nucl Med. 2009;50:477–84.
Plyku D, Hobbs RF, Huang K, et al. Recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal in 124I-PET/CT based dosimetry for 131I therapy of metastatic differentiated thyroid cancer. J Nucl Med. 2017;58:1146–54.
ICRP. Basic anatomical and physiological data for use in radiological protection: reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. Ann ICRP. 2002;32:5–265.
SAAM II User Guide. Seattle: SAAM Institute, University of Washington; 1997.
Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the task group on reference man. ICRP publication 23. Elmsford, NY: International Commission on Radiological Protection; 1975.
ICRP. 1990 Recommendations of the International Commission on Radiological Protection. ICRP publication 60. Ann ICRP. 1991;21(1-3)
Thomas SR, Stabin MG, Chen CT, Samaratunga RC. MIRD Pamphlet No. 14: a dynamic urinary bladder model for radiation dose calculations. J Nucl Med. 2012;33:783–802.
Agostinelli S, Allison J, Amako KA, et al. GEANT4 – a simulation toolkit. Nucl Instrum Methods Phys Res A. 2003;506:250–303.
Tamboli DA, Harris MA, Hogg JP, Realini T, Sivak-Callcott JA. Computed tomography dimensions of the lacrimal gland in normal Caucasian orbits. Ophthalmic Plast Reconstr Surg. 2011;27:453–6.
Price KM, Richard MJ. Ophthalmic Pearls: The tearing patient: diagnosis and management. EyeNet Magazine, 2009; p. 33–5.
Montés-Micó R. Role of the tear film in the optical quality of the human eye. J Cataract Refract Surg. 2007;33:1631–5.
Sgouros G, Frey E, Wahl R, He B, Prideaux A, Hobbs R. Three-dimensional imaging-based radiobiological dosimetry. Semin Nucl Med. 2008;38:321–34.
Prideaux AR, Song H, Hobbs RF, et al. Three-dimensional radiobiologic dosimetry: application of radiobiologic modeling to patient-specific 3-dimensional imaging-based internal dosimetry. J Nucl Med. 2007;48:1008–16.
Sgouros G, Hobbs RF, Atkins FB, Van Nostrand D, Ladenson PW, Wahl RL. Three-dimensional radiobiological dosimetry (3D-RD) with 124I PET for 131I therapy of thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38(Suppl 1):S41–7.
Hobbs RF, Wahl RL, Lodge MA, et al. 124I PET-Based 3D-RD dosimetry for a pediatric thyroid cancer patient: real time treatment planning and methodologic comparison. J Nucl Med. 2009; 50(11):1844–47.
King-Smith PE, Fink BA, Hill RM, Koelling KW, Tiffany JM. The thickness of the tear film. Curr Eye Res. 2004;29:357–68.
Pfob CH, Ziegler S, Graner FP, et al. Biodistribution and radiation dosimetry of 68Ga-PSMA HBED CC – a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1962–70.
Herrmann K, Bluemel C, Weineisen M, et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J Nucl Med. 2015;56:855–61.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–705.
Okamoto S, Thieme A, Allmann J, et al. Radiation dosimetry for 177Lu-PSMA I&T in metastatic castration-resistant prostate cancer: absorbed dose in normal organs and tumor lesions. J Nucl Med. 2017;58:445–50.
Hohberg M, Eschner W, Schmidt M, et al. Lacrimal glands may represent organs at risk for radionuclide therapy of prostate cancer with [(177)Lu]DKFZ-PSMA-617. Mol Imaging Biol. 2016;18:437–45.
Acknowledgments
We acknowledge the support of the Prostate Cancer Foundation – Young Investigator Award, the Patrick C. Walsh Prostate Cancer Research Fund, EB006351, CA134675, CA184228, CA103175, CA183031 and CA116477.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plyku, D., Mena, E., Rowe, S.P. et al. Combined model-based and patient-specific dosimetry for 18F-DCFPyL, a PSMA-targeted PET agent. Eur J Nucl Med Mol Imaging 45, 989–998 (2018). https://doi.org/10.1007/s00259-018-3939-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-3939-x