Abstract
Purpose
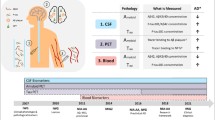
Cerebral glucose metabolism measured with [18F]-FDG PET is a well established marker of neuronal dysfunction in neurodegeneration. The tau-protein tracer [18F]-AV-1451 PET is currently under evaluation and shows promising results. Here, we assess the feasibility of early perfusion imaging with AV-1451 as a substite for FDG PET in assessing neuronal injury.
Methods
Twenty patients with suspected neurodegeneration underwent FDG and early phase AV-1451 PET imaging. Ten one-minute timeframes were acquired after application of 200 MBq AV-1451. FDG images were acquired on a different date according to clinical protocol. Early AV-1451 timeframes were coregistered to individual FDG-scans and spatially normalized. Voxel-wise intermodal correlations were calculated on within-subject level for every possible time window. The window with highest pooled correlation was considered optimal. Z-transformed deviation maps (ZMs) were created from both FDG and early AV-1451 images, comparing against FDG images of healthy controls.
Results
Regional patterns and extent of perfusion deficits were highly comparable to metabolic deficits. Best results were observed in a time window from 60 to 360 s (r = 0.86). Correlation strength ranged from r = 0.96 (subcortical gray matter) to 0.83 (frontal lobe) in regional analysis. ZMs of early AV-1451 and FDG images were highly similar.
Conclusion
Perfusion imaging with AV-1451 is a valid biomarker for assessment of neuronal dysfunction in neurodegenerative diseases. Radiation exposure and complexity of the diagnostic workup could be reduced significantly by routine acquisition of early AV-1451 images, sparing additional FDG PET.




Similar content being viewed by others
References
Bischof GN, Jessen F, Fliessbach K, et al. Impact of tau and amyloid burden on glucose metabolism in Alzheimer’s disease. Ann Clin Transl Neurol. 2016;3:934–9.
Dronse J, Fliessbach K, Bischof GN, et al. In vivo Patterns of Tau Pathology, Amyloid-β Burden, and Neuronal Dysfunction in Clinical Variants of Alzheimer’s Disease. J Alzheimers Dis. 2016:1–7.
Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–9.
Spina S, Schonhaut DR, Boeve BF, et al. Frontotemporal dementia with the V337M MAPT mutation: Tau-PET and pathology correlations. Neurology. 2017;88:758–66.
Dani M, Brooks DJ, Edison P. Tau imaging in neurodegenerative diseases. Eur J Nucl Med Mol Imaging. 2016;43:1139–50.
Hammes J, Bischof GN, Giehl K, et al. Elevated in vivo [18F]-AV-1451 uptake in a patient with progressive supranuclear palsy. Mov Disord. 2017;32:170–1.
Cho H, Choi JY, Hwang MS, et al. Subcortical (18) F-AV-1451 binding patterns in progressive supranuclear palsy. Mov Disord. 2017;32:134–40.
Whitwell JL, Lowe VJ, Tosakulwong N, et al. [18F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord. 2017;32:124–33.
Smith R, Schain M, Nilsson C, et al. Increased basal ganglia binding of (18) F-AV-1451 in patients with progressive supranuclear palsy. Mov Disord. 2017;32:108–14.
Betthauser T, Lao PJ, Murali D, et al. In vivo comparison of tau radioligands 18F-THK-5351 and 18F-THK-5317. J Nucl Med. 2017;58:996–1002.
Xia C-F, Arteaga J, Chen G, et al. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9:666–76.
Walji AM, Hostetler ED, Selnick H, et al. Discovery of 6-(Fluoro-(18)F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([(18)F]-MK-6240): A positron emission tomography (PET) imaging agent for quantification of neurofibrillary tangles (NFTs). J Med Chem. 2016;59:4778–89.
van Eimeren T, Bischof GN, Drzezga AE. Is tau imaging more than just ‘upside-down’ FDG imaging? J Nucl Med; Epub ahead of print 10 May 2017. https://doi.org/10.2967/jnumed.117.190082.
Ng KP, Pascoal TA, Mathotaarachchi S, et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F–THK5351 uptake in the human brain. Alzheimers Res Ther; 9. Epub ahead of print 31 March 2017. https://doi.org/10.1186/s13195-017-0253-y.
Saint-Aubert L, Lemoine L, Chiotis K, et al. Tau PET imaging: present and future directions. Mol Neurodegener. 2017;12:19.
Teipel S, Drzezga A, Grothe MJ, et al. Multimodal imaging in Alzheimer’s disease: validity and usefulness for early detection. Lancet Neurol. 2015;14:1037–53.
Heiss WD, Herholz K, Pawlik G, et al. Positron emission tomography as a quantitative imaging method for demonstrating regional brain metabolism. Digitale Bilddiagn. 1984;4:37–45.
Phillips AA, Chan FH, Zheng MMZ, et al. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab. 2016;36:647–64.
Mielke R, Pietrzyk U, Jacobs A, et al. HMPAO SPET and FDG PET in Alzheimer’s disease and vascular dementia: comparison of perfusion and metabolic pattern. Eur J Nucl Med. 1994;21:1052–60.
Tiepolt S, Hesse S, Patt M, et al. Early [18F]florbetaben and [11C]PiB PET images are a surrogate biomarker of neuronal injury in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2016;43:1700–9.
Rostomian AH, Madison C, Rabinovici GD, et al. Early 11C-PIB frames and 18F-FDG PET measures are comparable: A study validated in a cohort of AD and FTLD patients. J Nucl Med. 2011;52:173–9.
Meyer PT, Hellwig S, Amtage F, et al. Dual-biomarker imaging of regional cerebral amyloid load and neuronal activity in dementia with PET and 11C-labeled Pittsburgh compound B. J Nucl Med. 2011;52:393–400.
Lin K-J, Hsiao I-T, Hsu J-L, et al. Imaging characteristic of dual-phase 18F-florbetapir (AV-45/Amyvid) PET for the concomitant detection of perfusion deficits and beta-amyloid deposition in Alzheimer’s disease and mild cognitive impairment. Eur J Nucl Med Mol Imaging. 2016;43:1304–14.
Hsiao I-T, Huang C-C, Hsieh C-J, et al. Correlation of early-phase 18F-florbetapir (AV-45/Amyvid) PET images to FDG images: preliminary studies. Eur J Nucl Med Mol Imaging. 2012;39:613–20.
Jin S, Oh M, Oh SJ, et al. Additional value of early-phase 18F-FP-CIT PET image for differential diagnosis of atypical parkinsonism. Clin Nucl Med. 2017;42:e80–7.
Verfaillie SCJ, Adriaanse SM, Binnewijzend MAA, et al. Cerebral perfusion and glucose metabolism in Alzheimer’s disease and frontotemporal dementia: two sides of the same coin? Eur Radiol. 2015;25:3050–9.
Rodriguez-Vieitez E, Leuzy A, Chiotis K, et al. Comparability of [18F]THK5317 and [11C]PIB blood flow proxy images with [18F]FDG positron emission tomography in Alzheimer’s disease. J Cereb Blood Flow Metab. 2016; 0271678X16645593
Evans AC, Collins DL, Mills SR, et al. 3D statistical neuroanatomical models from 305 MRI volumes. In: Nuclear Science Symposium and Medical Imaging Conference, 1993., 1993 I.E. Conference Record. 1993, pp. 1813–1817 vol.3.
Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1915;10:507–21.
Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–47.
Makris N, Goldstein JM, Kennedy D, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83:155–71.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89.
Minoshima S, Frey KA, Koeppe RA, et al. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of Fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–48.
Vollmar S, Čížek J, Sué M, et al. VINCI-volume imaging in neurological research, co-registration and ROIs included. In: Kremer K, Macho V, editors. Forschung und wissenschaftliches Rechnen 2003. Göttingen: GWDG; 2004. p. 115–31.
Wong CO, Thie J, Gaskill M, et al. A statistical investigation of normal regional intra-subject heterogeneity of brain metabolism and perfusion by F-18 FDG and O-15 H2O PET imaging. BMC Nucl Med. 2006;6:4.
Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–67.
Schonhaut DR, Ossenkoppele R, Bejanin A, et al. Tau-pet patterns overlap and exceed hypometabolism in Alzheimer’s disease. Alzheimers Dement. 2016;12:545–P547.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
IL, GNB and JH report no potential conflicts of interest. AD received consulting and speaker honoraria and research support from Siemens Healthcare, AVID Radiopharmaceuticals, Lilly, Piramal, and GE Healthcare. TvE received lecture fees from Lilly Germany, and research funding from the German Research Foundation (DFG), the Leibniz Association and the EU-joint program for neurodegenerative disease research (JPND).
Rights and permissions
About this article
Cite this article
Hammes, J., Leuwer, I., Bischof, G.N. et al. Multimodal correlation of dynamic [18F]-AV-1451 perfusion PET and neuronal hypometabolism in [18F]-FDG PET. Eur J Nucl Med Mol Imaging 44, 2249–2256 (2017). https://doi.org/10.1007/s00259-017-3840-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3840-z