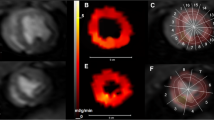
Abstract
Purpose
18F-labeled myocardial flow agents are becoming available for clinical application but the ∼2 hour half-life of 18F complicates their clinical application for rest-stress measurements. The goal of this work is to evaluate in a pig model a single-scan method which provides quantitative rest-stress blood flow in less than 15 minutes.
Methods
Single-scan rest-stress measurements were made using 18F-Flurpiridaz. Nine scans were performed in healthy pigs and seven scans were performed in injured pigs. A two-injection, single-scan protocol was used in which an adenosine infusion was started 4 minutes after the first injection of 18F-Flurpiridaz and followed either 3 or 6 minutes later by a second radiotracer injection. In two pigs, microsphere flow measurements were made at rest and during stress. Dynamic images were reoriented into the short axis view, and regions of interest (ROIs) for the 17 myocardial segments were defined in bull’s eye fashion. PET data were fitted with MGH2, a kinetic model with time varying kinetic parameters, in which blood flow changes abruptly with the introduction of adenosine. Rest and stress myocardial blood flow (MBF) were estimated simultaneously.
Results
The first 12–14 minutes of rest-stress PET data were fitted in detail by the MGH2 model, yielding MBF measurement with a mean precision of 0.035 ml/min/cc. Mean myocardial blood flow across pigs was 0.61 ± 0.11 mL/min/cc at rest and 1.06 ± 0.19 mL/min/cc at stress in healthy pigs and 0.36 ± 0.20 mL/min/cc at rest and 0.62 ± 0.24 mL/min/cc at stress in the ischemic area. Good agreement was obtained with microsphere flow measurement (slope = 1.061 ± 0.017, intercept = 0.051 ± 0.017, mean difference 0.096 ± 0.18 ml/min/cc).
Conclusion
Accurate rest and stress blood flow estimation can be obtained in less than 15 min of PET acquisition. The method is practical and easy to implement suggesting the possibility of clinical translation.







Similar content being viewed by others
References
Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005;46:75–88.
Krivokapich J, Smith GT, Huang SC, et al. 13N ammonia myocardial imaging at rest and with exercise in normal volunteers. Quantification of absolute myocardial perfusion with dynamic positron emission tomography. Circulation. 1989;80:1328–37.
Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–80.
Blanksma PK, Willemsen AT, Meeder JG, et al. Quantitative myocardial mapping of perfusion and metabolism using parametric polar map displays in cardiac PET. J Nucl Med. 1995;36:153–8.
Stewart RE, Schwaiger M, Molina E, et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol. 1991;67:1303–10.
Knaapen P. Quantitative myocardial blood flow imaging: not all flow is equal. Eur J Nucl Med Mol Imaging. 2014;41:116–8.
Danad I, Raijmakers PG, Appelman YE, et al. Coronary risk factors and myocardial blood flow in patients evaluated for coronary artery disease: a quantitative [15O]H2O PET/CT study. Eur J Nucl Med Mol Imaging. 2012;39:102–12.
Lortie M, Beanlands RS, Yoshinaga K, et al. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007;34:1765–74.
Camici PG, Rimoldi OE. A novel (18)F-labeled tracer for the quantification of myocardial blood flow and infarct size with positron-emission tomography: Another way to avoid the need of an on-site cyclotron. Circ Cardiovasc Imaging. 2009;2:75–6.
Nekolla SG, Saraste A. Novel F-18-labeled PET myocardial perfusion tracers: bench to bedside. Curr Cardiol Rep. 2011;13:145–50.
Rust TC, DiBella EV, McGann CJ, et al. Rapid dual-injection single-scan 13N-ammonia PET for quantification of rest and stress myocardial blood flows. Phys Med Biol. 2006;51:5347–62.
Alpert N, Dean Fang YH, El Fakhri G. Single-scan rest/stress imaging 18F-labeled flow tracers. Med Phys. 2012;39:6609–20.
Yalamanchili P, Wexler E, Hayes M, et al. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14:782–8.
Huisman MC, Higuchi T, Reder S, et al. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49:630–6.
Nekolla SG, Reder S, Saraste A, et al. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation. 2009;119:2333–42.
Packard RR, Huang SC, Dahlbom M, et al. Absolute quantification of myocardial blood flow in human subjects with or without myocardial ischemia using dynamic flurpiridaz F18 PET. J Nucl Med. 2014;55:1438–44.
Maddahi J, Czernin J, Lazewatsky J, et al. Phase I, first-in-human study of BMS747158, a novel 18F-labeled tracer for myocardial perfusion PET: dosimetry, biodistribution, safety, and imaging characteristics after a single injection at rest. J Nucl Med. 2011;52:1490–98.
Heymann MA, Payne BD, Hoffman JI, et al. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977;20:55–79.
Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healtcare professionals from the Cardiac Imaging Committe of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovascular Imaging. 2002;18:539–42.
Reinhardt CP, Dalhberg S, Tries MA, et al. Stable labeled microspheres to measure perfusion: validation of a neutron activation assay technique. Am J Physiol Heart Circ Physiol 2001;280:H108:16.
Wilson RF, Wyche K, Christensen BV, et al. Effect of adenosine on human coronary arterial circulation. Circulation. 1990;82:1595–606.
Rossen JD, Quillen JE, Lopez AG, et al. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18:485–91.
Kern MJ, Deligonul U, Tatineni S, et al. Intravenous adenosine: continuous infusion and low dose bolus administration for determination of coronary vasodilator reserve in patients with and without coronary artery disease. J Am Coll Cardiol. 1991;18:718–29.
Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol Rev. 1990;70:761–845.
Sherif HM, Nekolla SG, Saraste A, et al. Simplified quantification of myocardial flow reserve with flurpiridaz F18: validation with microspheres in a pig model. J Nucl Med. 2011;52:617–24.
Johnson NP, Gould KL. Simplified quantification of myocardial flow reserve with (18)F-flurpiridaz: validation with microspheres in a pig model. J Nucl Med. 2011;52:1835–6.
Acknowledgements
This work was supported by the following grant: R01HL110241. The authors thank Kevin Cordaro, Victoria Douglas and Julia Scotton for animal preparation, handling and monitoring and Dr. Moses Wilks and Dr. Eline Verwer for their help during experimental measurements. The authors are also very grateful to Dr Moussa Mansour for letting them use his facility for the pig preparation and surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by R01HL110241.
Conflict of interest
Nicolas J. Guehl declares that he has no conflict of interest. Marc D. Normandin declares that he has no conflict of interest. Dustin W. Wooten declares that he has no conflict of interest. Guy Rozen declares that he has no conflict of interest. Arkadiusk Sitek declares that he has no conflict of interest. Jeremy Ruskin declares that he has no conflict of interest. Timothy M. Shoup declares that he has no conflict of interest. Leon M. Ptaszek declares that he has no conflict of interest. Georges El Fakhri declares that he has no conflict of interest. Nathaniel M. Alpert declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Nicolas J. Guehl and Marc D. Normandin contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Arterial whole blood sample measurements (ART WB) and PET measurements in the LV chamber. (JPG 176 kb)
Supplemental Fig. 2
Relative error on the estimation of K 1 plotted against K 1 estimates for the 17 segments and all studies. The bold line corresponds to the fit of σ(K 1 )/K 1 = a /K1, where a was estimated to be 0.035. (JPG 3469 kb)
Supplemental Fig. 3
Standard perfusion images generated from the kinetic parameters. The top row shows the perfusion images at rest and the bottom row during stress. Left to right columns are short axis, horizontal axis and vertical axis views, respectively. (JPG 9255 kb)
Rights and permissions
About this article
Cite this article
Guehl, N.J., Normandin, M.D., Wooten, D.W. et al. Single-scan rest/stress imaging: validation in a porcine model with 18F-Flurpiridaz. Eur J Nucl Med Mol Imaging 44, 1538–1546 (2017). https://doi.org/10.1007/s00259-017-3684-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3684-6