Abstract
Purpose
Evidence for the prodromal stage of dementia with Lewy bodies (DLB) is very limited. To address this issue, we investigate the 123I-FP-CIT SPECT measure of dopamine transporter binding finding and its clinical relevance.
Methods
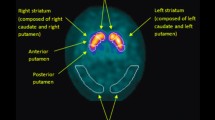
We enrolled subjects into a prodromal DLB group (PRD-DLB) (n = 20) and clinical DLB group (CLIN-DLB) (n = 18) and compared these groups with an Alzheimer’s disease control group (AD) (n = 10). PRD-DLB was defined as patients having the non-motor symptoms associated with Lewy body disease (LBD) [i.e. REM sleep behavior disorder (RBD), olfactory dysfunction, autonomic dysfunction, and depression] and showing characteristic diffuse occipital hypometabolism in 18F-FDG PET. CLIN-DLB was defined as patients fulfilling the established criteria of probable DLB. Striatal specific binding ratio (SBR) of 123I-FP-CIT SPECT was used for objective group comparisons. The correlations between SBR and cognitive function (MMSE), motor symptoms (UPDRS3), and duration of LBD-associated non-motor symptoms were compared between the two DLB groups.
Results
Mean SBR scores of both PRD-DLB and CLIN-DLB were significantly lower than those of AD. No correlation was found between SBR and MMSE scores. Both in the CLIN-DLB and total DLB groups, SBR scores were negatively correlated with UPDRS3 scores, whereas no correlation was found in PRD-DLB. Among the LBD-related non-motor symptoms, duration of olfactory dysfunction, and RBD demonstrated negative correlation with SBR scores in PRD-DLB.
Conclusion
123I-FP-CIT SPECT may play a role for detecting DLB among the subjects in prodromal stage. During this stage, long-term olfactory dysfunction and/or RBD may indicate more severe degeneration of the nigro-striatal dopaminergic pathway.

Similar content being viewed by others
References
McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72.
Chaudhuri KR, Martinez-Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord. 2006;21:916–23.
Chiba Y, Fujishiro H, Iseki E, et al. Retrospective survey of prodromal symptoms in dementia with Lewy bodies: comparison with Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33:273–81.
Papathanasiou ND, Boutsiadis A, Dickson J, Bomanji JB. Diagnostic accuracy of 123I-FP-CIT (DaTSCAN) in dementia with Lewy bodies: a meta-analysis of published studies. Parkinsonism Relat Disord. 2012;18:225–9.
Fujishiro H, Iseki E, Kasanuki K, et al. Glucose hypometabolism in primary visual cortex is commonly associated with clinical features of dementia with Lewy bodies regardless of cognitive conditions. Int J Geriatr Psychiatr. 2012;27:1138–46.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Int Med;256:183–194.
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in ‘probable’ Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–72.
Fazekas F, Kleinert R, Offenbacher H, et al. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. Am J Neuroradiol. 1991;12:915–21.
E. Iseki, N. Murayama, R. Yamamoto, H, et al. Construction of a (18)F-FDG PET normative database of Japanese healthy elderly subjects and its application to demented and mild cognitive impairment patients. Int J Geriatr Psychiatry. 2010; 25: 352–361.
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional Stereotactic surface projections of Fluorine-19-FDG PET. J Nucl Med. 1995;36:1238–48.
Mizumura S, Kumita S, Cho K, et al. Development of quantitative analysis method for stereotactic brain image: assessment of reduced accumulation in extent and severity using anatomical segmentation. Ann Nucl Med. 2003;17:289–95.
Japanese society of nuclear medicine. Japanese ioflupane guideline working group recommendation. http://www.jsnm.org/guideline/ioflupane. Accessed 3 Jun 2016.
Kuya K, Shinohara Y, Miyoshi F, Fujii S, Tanabe Y, Ogawa T. Correlation between neuromelanin-sensitive MR imaging and 123I-FP-CIT SPECT in patients with parkinsonism. Neuroradiology. 2016;58:351–6.
Dickson JC, Tossici-Bolt L, Sera T, et al. The impact of reconstruction method on the quantification of DaTSCAN images. Eur J Nucl Med Mol Imaging. 2010;37:23–35.
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I] FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33:1491–9.
Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54.
Tolosa E, Borght TV, Moreno E. Accuracy of DaTSCAN (123I-Ioflupane) SPECT in diagnosis of patients with clinically uncertain parkinsonism: 2-year follow-up of an open-label study. Mov Disord. 2007;22:2346–51.
Siepel FJ, Rongve A, Buter TC, et al. FP-CIT SPECT in suspected dementia with Lewy bodies: A longitudinal case study. BMJ Open. 123I. doi:10.1136/bmjopen-2013-002642.
Fujishiro H, Iseki E, Murayama N, et al. Diffuse occipital hypometabolism on [18F]-FDG PET scans in patients with idiopathic REM sleep behavior disorder: Prodomal dementia with Lewy bodies? Psychogeriatrics. 2010;10:144–52.
Fujishiro H, Iseki E, Kasanuki K, et al. A follow up study of non-demented patients with primary visual cortical hypometabolism: prodromal dementia with Lewy bodies. J Neurol Sci. 2013;334:48–54.
Bohnen NI, Koeppe RA, Minoshima S, et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J Nucl Med. 2011;52:848–55.
Booij J, Tissingh G, Boer GJ, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson's disease. J Neurol Neurosurg Psych. 1997;62:133–40.
Rektorova I, Srovnalova H, Kubikova R, Prasek J. Striatal dopamine transporter imaging correlates with depressive symptoms and tower of London task performance in Parkinson’s disease. Mov Disord. 2008;23:1580–7.
Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–22.
Burruss JW, Hurley RA, Taber KH, Rauch RA, Norton RE, Hayman LA. Functional neuroanatomy of the frontal lobe circuits. Radiology. 2000;214:227–30.
Ziebell M, Andersen BB, Pinborg LH, et al. Striatal dopamine transporter binding does not correlate with clinical severity in dementia with Lewy bodies. J Nucl Med. 2013;54:1072–6.
Del Sole A, Perini G, Lecchi M, Mariani C, Lucignani G, Clerici F. Correlation between 123I-FP-CIT brain SPECT and parkinsonism in dementia with Lewy bodies: caveat for clinical use. Clin Nucl Med. 2015;40:32–5.
Roselli F, Pisciotta NM, Perneczky R, et al. Severity of neuropsychiatric symptoms and dopamine transporter levels in dementia with Lewy bodies: A 123I-FP-CIT SPECT study. Mov Disord. 2009;24:2097–103.
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Marsden CD. Parkinson’s disease. Lancet. 1990;335:948–52.
Attems J, Quass M, Jellinger KA. Tau and alpha-synuclein brainstem pathology in Alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol. 2007;113:53–62.
Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78:1176–81.
Taylor JP, Colloby SJ, McKeith IG, et al. Cholinesterase inhibitor use does not significantly influence the ability of 123I-FP-CIT imaging to distinguish Alzheimer’s disease from dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2007;78:1069–71.
Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–61.
Acknowledgments
None authors declare that they have conflicts of interest. The present study was supported, in part, by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (grant number: 90648859).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasanuki, K., Iseki, E., Ota, K. et al. 123I-FP-CIT SPECT findings and its clinical relevance in prodromal dementia with Lewy bodies. Eur J Nucl Med Mol Imaging 44, 358–365 (2017). https://doi.org/10.1007/s00259-016-3466-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3466-6