Abstract
Purpose
Somatostatin-based radiopeptide treatment is generally performed using the β-emitting radionuclides 90Y or 177Lu. The present study aimed at comparing benefits and harms of both therapeutic approaches.
Methods
In a comparative cohort study, patients with advanced neuroendocrine tumours underwent repeated cycles of [90Y-DOTA]-TOC or [177Lu-DOTA]-TOC until progression of disease or permanent adverse events. Multivariable Cox regression and competing risks regression were employed to examine predictors of survival and adverse events for both treatment groups.
Results
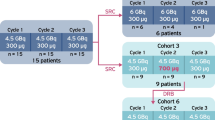
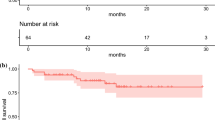
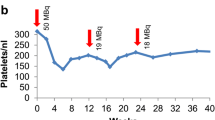
Overall, 910 patients underwent 1,804 cycles of [90Y-DOTA]-TOC and 141 patients underwent 259 cycles of [177Lu-DOTA]-TOC. The median survival after [177Lu-DOTA]-TOC and after [90Y-DOTA]-TOC was comparable (45.5 months versus 35.9 months, hazard ratio 0.91, 95 % confidence interval 0.63–1.30, p = 0.49). Subgroup analyses revealed a significantly longer survival for [177Lu-DOTA]-TOC over [90Y-DOTA]-TOC in patients with low tumour uptake, solitary lesions and extra-hepatic lesions. The rate of severe transient haematotoxicities was lower after [177Lu-DOTA]-TOC treatment (1.4 vs 10.1 %, p = 0.001), while the rate of severe permanent renal toxicities was similar in both treatment groups (9.2 vs 7.8 %, p = 0.32).
Conclusion
The present results revealed no difference in median overall survival after [177Lu-DOTA]-TOC and [90Y-DOTA]-TOC. Furthermore, [177Lu-DOTA]-TOC was less haematotoxic than [90Y-DOTA]-TOC.




Similar content being viewed by others
References
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26(18):3063–72.
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364(6):501–13.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364(6):514–23.
Oberg K. Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol 2001;12 Suppl 2:S111–4.
Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 2003;24(4):389–427.
Oberg K. Cancer: antitumor effects of octreotide LAR, a somatostatin analog. Nat Rev Endocrinol 2010;6(4):188–9.
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27(28):4656–63.
Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989;1(8632):242–4.
Otte A, Mueller-Brand J, Dellas S, Nitzsche EU, Herrmann R, Maecke HR. Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet 1998;351(9100):417–8.
Marincek N, Jörg AC, Brunner P, Schindler C, Koller MT, Rochlitz C, et al. Somatostatin-based radiotherapy with [90Y-DOTA]-TOC in neuroendocrine tumors: long-term outcome of a phase I dose escalation study. J Transl Med 2013;11:17.
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29(17):2416–23.
Otte A, Jermann E, Behe M, Goetze M, Bucher HC, Roser HW, et al. DOTATOC: a powerful new tool for receptor-mediated radionuclide therapy. Eur J Nucl Med 1997;24(7):792–5.
Villard L, Romer A, Marincek N, Brunner P, Koller MT, Schindler C, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol 2012;30(10):1100–6.
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999;26(11):1439–47.
Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 2001;12(7):941–5.
Iten F, Müller B, Schindler C, Rochlitz C, Oertli D, Mäcke HR, et al. Response to [90Yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res 2007;13(22 Pt 1):6696–702.
Iten F, Muller B, Schindler C, Rasch H, Rochlitz C, Oertli D, et al. [(90)Yttrium-DOTA]-TOC response is associated with survival benefit in iodine-refractory thyroid cancer: long-term results of a phase 2 clinical trial. Cancer 2009;115(10):2052–62.
Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994;330(13):877–84.
de Jong M, Breeman WA, Valkema R, Bernard BF, Krenning EP. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med 2005;46 Suppl 1:13S–7S.
Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26(11):2389–430.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92(3):205–16.
Siegel JA, Stabin MG. Absorbed fractions for electrons and beta particles in spheres of various sizes. J Nucl Med 1994;35(1):152–6.
Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340:c117.
Walrand S, Barone R, Pauwels S, Jamar F. Experimental facts supporting a red marrow uptake due to radiometal transchelation in 90Y-DOTATOC therapy and relationship to the decrease of platelet counts. Eur J Nucl Med Mol Imaging 2011;38(7):1270–80.
Chen PS, Terepka AR, Hodge HC. The pharmacology and toxicology of the bone seekers. Annu Rev Pharmacol 1961;1(1):369–96.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26(13):2124–30.
Acknowledgments
We are grateful to all patients and referring institutions for participation. Furthermore, we thank our nursing, laboratory and technical staff for their support. Lastly, we are grateful for the input of Thomas Krause on the manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Romer and D. Seiler contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table A
Hazard ratios for overall survival 1, 2 and 5 years after start of DOTA-TOC therapy (n=1051) (DOC 52 kb)
Rights and permissions
About this article
Cite this article
Romer, A., Seiler, D., Marincek, N. et al. Somatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 41, 214–222 (2014). https://doi.org/10.1007/s00259-013-2559-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2559-8