Abstract
Purpose
In disseminated prostate cancer, expression of human epidermal growth factor receptor type 2 (HER2) is one of the pathways to androgen independence. Radionuclide molecular imaging of HER2 expression in disseminated prostate cancer might identify patients for HER2-targeted therapy. Affibody molecules are small (7 kDa) targeting proteins with high potential as tracers for radionuclide imaging. The goal of this study was to develop an optimal Affibody-based tracer for visualization of HER2 expression in prostate cancer.
Methods
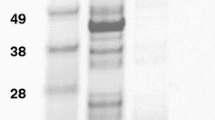
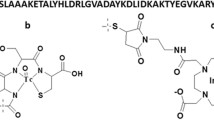
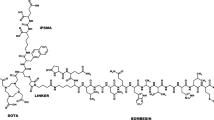
A synthetic variant of the anti-HER2 ZHER2:342 Affibody molecule, ZHER2:S1, was N-terminally conjugated with the chelators DOTA, NOTA and NODAGA. The conjugated proteins were biophysically characterized by electrospray ionization mass spectroscopy (ESI-MS), circular dichroism (CD) spectroscopy and surface plasmon resonance (SPR)-based biosensor analysis. After labelling with 111In, the biodistribution was assessed in normal mice and the two most promising conjugates were further evaluated for tumour targeting in mice bearing DU-145 prostate cancer xenografts.
Results
The HER2-binding equilibrium dissociation constants were 130, 140 and 90 pM for DOTA-ZHER2:S1, NOTA-ZHER2:S1 and NODAGA-ZHER2:S1, respectively. A comparative study of 111In-labelled DOTA-ZHER2:S1, NOTA-ZHER2:S1 and NODAGA-ZHER2:S1 in normal mice demonstrated a substantial influence of the chelators on the biodistribution properties of the conjugates. 111In-NODAGA-ZHER2:S1 had the most rapid clearance from blood and healthy tissues. 111In-NOTA-ZHER2:S1 showed high hepatic uptake and was excluded from further evaluation. 111In-DOTA-ZHER2:S1 and 111In-NODAGA-ZHER2:S1 demonstrated specific uptake in DU-145 prostate cancer xenografts in nude mice. The tumour uptake of 111In-NODAGA-ZHER2:S1, 5.6 ± 0.4%ID/g, was significantly lower than the uptake of 111In-DOTA-ZHER2:S1, 7.4 ± 0.5%ID/g, presumably because of lower bioavailability due to more rapid clearance. 111In-NODAGA-ZHER2:S1 provided higher tumour-to-blood ratio, but somewhat lower tumour-to-liver, tumour-to-spleen and tumour-to-bone ratios.
Conclusion
Since distant prostate cancer metastases are situated in bone or bone marrow, the higher tumour-to-bone ratio is the most important. This renders 111In-DOTA-ZHER2:S1 a preferable agent for imaging of HER2 expression in disseminated prostate cancer.





Similar content being viewed by others
References
Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst 2000;92:1918–25.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–37.
Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med 1999;5:280–5.
Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 1994;54:5474–8.
Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, White FM, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, Weber MJ. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem 2002;277:29304–14.
Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 2005;23:8253–61.
So A, Gleave M, Hurtado-Col A, Nelson C. Mechanisms of the development of androgen independence in prostate cancer. World J Urol 2005;23:1–9.
Minner S, Jessen B, Stiedenroth L, Burandt E, Köllermann J, Mirlacher M, Erbersdobler A, Eichelberg C, Fisch M, Brümmendorf TH, Bokemeyer C, Simon R, Steuber T, Graefen M, Huland H, Sauter G, Schlomm T. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res 2010;16:1553–60.
Bacus SS, Altomare DA, Lyass L, Chin DM, Farrell MP, Gurova K, Gudkov A, Testa JR. AKT2 is frequently upregulated in HER-2/neu-positive breast cancers and may contribute to tumor aggressiveness by enhancing cell survival. Oncogene 2002;21:3532–40.
Tolmachev V. Imaging of HER-2 overexpression in tumors for guiding therapy. Curr Pharm Des 2008;14:2999–3019.
Malmberg J, Tolmachev V, Orlova A. Imaging agents for in vivo molecular profiling of disseminated prostate cancer: cellular processing of [111In]-labeled CHX-A″ DTPA-trastuzumab and anti-HER2 ABY-025 Affibody in prostate cancer cell lines. Exp Ther Med 2011;2:523–8.
Löfblom J, Feldwisch J, Tolmachev V, Carlsson J, Ståhl S, Frejd FY. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett 2010;584:2670–80.
Ahlgren S, Tolmachev V. Radionuclide molecular imaging using Affibody molecules. Curr Pharm Biotechnol 2010;11:581–9.
Nygren PÅ. Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J 2008;275:2668–76.
Orlova A, Magnusson M, Eriksson T, Nilsson M, Larsson B, Höidén-Guthenberg I, Widström C, Carlsson J, Tolmachev V, Ståhl S, Nilsson FY. Tumor imaging using a picomolar affinity HER2 binding Affibody molecule. Cancer Res 2006;66:4339–48.
Baum RP, Prasad V, Müller D, Schuchardt C, Orlova A, Wennborg A, Tolmachev V, Feldwisch J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med 2010;51:892–7.
Malmberg J, Sandström M, Wester K, Tolmachev V, Orlova A. Comparative biodistribution of imaging agents for in vivo molecular profiling of disseminated prostate cancer (PC) in mice bearing PC xenografts: focus on 111In- and 125I-labeled anti-HER2 humanized monoclonal trastuzumab and ABY-025 Affibody. Nucl Med Biol 2011. doi:10.1016/j.nucmedbio.
Engfeldt T, Orlova A, Tran T, Bruskin A, Widström C. Eriksson Karlström A, Tolmachev V. Imaging of HER2-expressing tumours using a synthetic Affibody molecule containing the 99mTc-chelating mercaptoacetyl-glycyl-glycyl-glycyl (MAG3) sequence. Eur J Nucl Med Mol Imaging 2007;34:722–33.
Engfeldt T, Tran T, Orlova A, Widström C, Eriksson Karlström A, Tolmachev V. 99mTc-chelator engineering to improve tumour targeting properties of a HER2-specific Affibody molecule. Eur J Nucl Med Mol Imaging 2007;34:1843–53.
Tran T, Engfeldt T, Orlova A, Sandström M, Feldwisch J, Abrahmsén L, Wennborg A, Tolmachev V, Eriksson Karlström A. (99m)Tc-maEEE-Z(HER2:342), an Affibody molecule-based tracer for detection of HER2 expression in malignant tumors. Bioconjug Chem 2007;18:1956–64.
Tran T, Ekblad T, Orlova A, Sandström M, Feldwisch J, Wennborg A, Abrahmsén L, Tolmachev V, Eriksson Karlström A. Effects of lysine-containing mercaptoacetyl-based chelators on the biodistribution of 99mTc-labeled anti-HER2 Affibody molecules. Bioconjug Chem 2008;19:2568–76.
Ekblad T, Tran T, Orlova A, Widström C, Feldwisch J, Abrahmsén L, Wennborg A, Eriksson Karlström A, Tolmachev V. Development and preclinical characterisation of 99mTc-labelled Affibody molecules with reduced renal uptake. Eur J Nucl Med Mol Imaging 2008;35:2245–55.
Wållberg H, Orlova A, Altai M, Hosseinimehr SJ, Widström C, Malmberg J, Ståhl S, Tolmachev V. Molecular design and optimization of 99mTc-labeled recombinant affibody molecules improves their biodistribution and imaging properties. J Nucl Med 2011;52:461–9.
Tolmachev V, Altai M, Sandström M, Perols A, Eriksson Karlström A, Boschetti F, Orlova A. Evaluation of a maleimido derivative of NOTA for site-specific labeling of Affibody molecules. Bioconjug Chem 2011;22:894–902.
De León-Rodríguez LM, Kovacs Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjug Chem 2008;19:391–402.
Eisenwiener KP, Prata MI, Buschmann I, Zhang HW, Santos AC, Wenger S, Reubi JC, Mäcke HR. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjug Chem 2002;13:530–41.
Engfeldt T, Renberg B, Brumer H, Nygren PÅ, Eriksson Karlström A. Chemical synthesis of triple-labelled three-helix bundle binding proteins for specific fluorescent detection of unlabelled protein. Chembiochem 2005;6:1043–50.
Ross JS, Fletcher JA, Bloom KJ, Linette GP, Stec J, Symmans WF, Pusztai L, Hortobagyi GN. Targeted therapy in breast cancer: the HER-2/neu gene and protein. Mol Cell Proteomics 2004;3:379–98.
Persson M, Tolmachev V, Andersson K, Gedda L, Sandström M, Carlsson J. [(177)Lu]pertuzumab: experimental studies on targeting of HER-2 positive tumour cells. Eur J Nucl Med Mol Imaging 2005;32:1457–62.
Wållberg H, Orlova A. Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: implications for development of labeled tracers. Cancer Biother Radiopharm 2008;23:435–42.
Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res 2006;12:1665–71.
Nielsen DL, Andersson M, Kamby C. HER2-targeted therapy in breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Cancer Treat Rev 2009;35:121–36.
De Vita F, Giuliani F, Silvestris N, Catalano G, Ciardiello F, Orditura M. Human epidermal growth factor receptor 2 (HER2) in gastric cancer: a new therapeutic target. Cancer Treat Rev 2010;36 Suppl 3:S11–5.
Ziada A, Barqawi A, Glode LM, Varella-Garcia M, Crighton F, Majeski S, Rosenblum M, Kane M, Chen L, Crawford ED. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate 2004;60:332–7.
Lara Jr PN, Chee KG, Longmate J, Ruel C, Meyers FJ, Gray CR, Edwards RG, Gumerlock PH, Twardowski P, Doroshow JH, Gandara DR. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer 2004;100:2125–31.
Sridhar SS, Hotte SJ, Chin JL, Hudes GR, Gregg R, Trachtenberg J, Wang L, Tran-Thanh D, Pham NA, Tsao MS, Hedley D, Dancey JE, Moore MJ. A multicenter phase II clinical trial of lapatinib (GW572016) in hormonally untreated advanced prostate cancer. Am J Clin Oncol 2010;33:609–13.
Tolmachev V, Velikyan I, Sandström M, Orlova A. A HER2-binding Affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur J Nucl Med Mol Imaging 2010;37:1356–67.
Tolmachev V, Hofström C, Malmberg J, Ahlgren S, Hosseinimehr SJ, Sandström M, Abrahmsén L, Orlova A, Gräslund T. HEHEHE-tagged affibody molecule may be purified by IMAC, is conveniently labeled with [99(m)Tc(CO)3](+), and shows improved biodistribution with reduced hepatic radioactivity accumulation. Bioconjug Chem 2010;21:2013–22.
de Jong M, Bakker WH, Krenning EP, Breeman WA, van der Pluijm ME, Bernard BF, Visser TJ, Jermann E, Béhé M, Powell P, Mäcke HR. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTA0, d-Phe1, Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med 1997;24:368–71.
Froidevaux S, Heppeler A, Eberle AN, Meier AM, Häusler M, Beglinger C, Béhé M, Powell P, Mäcke HR. Preclinical comparison in AR4-2J tumor-bearing mice of four radiolabeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-somatostatin analogs for tumor diagnosis and internal radiotherapy. Endocrinology 2000;141:3304–12.
Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med 2004;45:116–23.
Decristoforo C, Hernandez Gonzalez I, Carlsen J, Rupprich M, Huisman M, Virgolini I, Wester HJ, Haubner R. 68Ga- and 111In-labelled DOTA-RGD peptides for imaging of alphavbeta3 integrin expression. Eur J Nucl Med Mol Imaging 2008;35:1507–15.
Acknowledgement
This study was supported by grants from the Swedish Cancer Society (Cancerfonden), the Swedish Research Council (Vetenskapsrådet) and the VINNOVA SAMBIO program.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jennie Malmberg and Anna Perols contributed equally to this study.
Rights and permissions
About this article
Cite this article
Malmberg, J., Perols, A., Varasteh, Z. et al. Comparative evaluation of synthetic anti-HER2 Affibody molecules site-specifically labelled with 111In using N-terminal DOTA, NOTA and NODAGA chelators in mice bearing prostate cancer xenografts. Eur J Nucl Med Mol Imaging 39, 481–492 (2012). https://doi.org/10.1007/s00259-011-1992-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1992-9