Abstract
Purpose
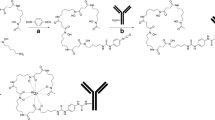
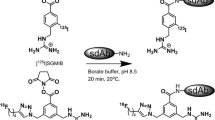
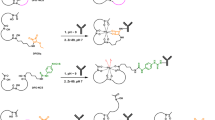
Improved bifunctional chelates (BFCs) are needed to facilitate efficient 64Cu radiolabeling of monoclonal antibodies (mAbs) under mild conditions and to yield stable, target-specific agents. The utility of two novel BFCs, 1-Oxa-4,7,10-triazacyclododecane-5-S-(4-isothiocyanatobenzyl)-4,7,10-triacetic acid (p-SCN-Bn-Oxo-DO3A) and 3,6,9,15-tetraazabicyclo[9.3.1]pentadeca-1(15),11,13-triene-4-S-(4-isothiocyanatobenzyl)-3,6,9-triacetic acid (p-SCN-Bn-PCTA), for mAb imaging with 64Cu were compared to the commonly used S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-tetraacetic acid (p-SCN-Bn-DOTA).
Methods
The BFCs were conjugated to trastuzumab, which targets the HER2/neu receptor. 64Cu radiolabeling of the conjugates was optimized. Receptor binding was analyzed using flow cytometry and radioassays. Finally, PET imaging and biodistribution studies were done in mice bearing either HER2/neu-positive or HER2/neu-negative tumors.
Results
64Cu-Oxo-DO3A- and PCTA-trastuzumab were prepared at room temperature in >95% radiochemical yield (RCY) in <30 min, compared to only 88% RCY after 2 h for the preparation of 64Cu-DOTA-trastuzumab under the same conditions. Cell studies confirmed that the immunoreactivity of the mAb was retained for each of the bioconjugates. In vivo studies showed that 64Cu-Oxo-DO3A- and PCTA-trastuzumab had higher uptake than the 64Cu-DOTA-trastuzumab at 24 h in HER2/neu-positive tumors, resulting in higher tumor to background ratios and better tumor images. By 40 h all three of the 64Cu-BFC-trastuzumab conjugates allowed for clear visualization of the HER2/neu-positive tumors but not the negative control tumor.
Conclusion
The antibody conjugates of PCTA and Oxo-DO3A were shown to have superior 64Cu radiolabeling efficiency and stability compared to the analogous DOTA conjugate. In addition, 64Cu-PCTA and Oxo-DO3A antibody conjugates may facilitate earlier imaging with greater target to background ratios than the analogous 64Cu-DOTA antibody conjugates.







Similar content being viewed by others
References
Wu AM. Antibodies and antimatter: the resurgence of immuno-PET. J Nucl Med 2009;50:2–5.
Barbet J, Kraeber-Bodéré F, Chatal JF. What can be expected from nuclear medicine tomorrow? Cancer Biother Radiopharm 2008;23:483–504.
Niu G, Li Z, Cao Q, Chen X. Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with (64)Cu-DOTA-trastuzumab. Eur J Nucl Med Mol Imaging 2009;36:1510–9.
Goldenberg DM. Targeted therapy of cancer with radiolabeled antibodies. J Nucl Med 2002;43:693–713.
Verel I, Visser GWM, van Dongen GA. The promise of immuno-PET in radioimmunotherapy. J Nucl Med 2005;46:164S–71S.
Ahlgren S, Wållberg H, Tran TA, Widström C, Hjertman M, Abrahmsén L, et al. Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J Nucl Med 2009;50:781–9.
Tang Y, Wang J, Scollard DA, Mondal H, Holloway C, Kahn HJ, et al. Imaging of HER2/neu-positive BT-474 human breast cancer xenografts in athymic mice using (111)In-trastuzumab (Herceptin) Fab fragments. Nucl Med Biol 2005;32:51–8.
Anderson CJ, Connett JM, Schwarz SW, Rocque PA, Guo LW, Philpott GW, et al. Copper-64-labeled antibodies for PET imaging. J Nucl Med 1992;33:1685–91.
Bryan JN, Jia F, Mohsin H, Sivaguru G, Miller WH, Anderson CJ, et al. Comparative uptakes and biodistributions of internalizing vs. noninternalizing copper-64 radioimmunoconjugates in cell and animal models of colon cancer. Nucl Med Biol 2005;32:851–8.
Bryan JN, Lewis MR, Henry CJ, Owen NK, Zhang J, Mohsin H, et al. Development of a two-antibody model for the evaluation of copper-64 radioimmunotherapy. Vet Comp Oncol 2004;2:82–90.
Cao Q, Cai W, Li Z-B, Chen K, He L, Li H-C, et al. PET imaging of acute and chronic inflammation in living mice. Eur J Nucl Med Mol Imaging 2007;34:1832–42.
Eiblmaier M, Meyer LA, Watson MA, Fracasso PM, Pike LJ, Anderson CJ. Correlating EGFR expression with receptor-binding properties and internalization of 64Cu-DOTA-cetuximab in 5 cervical cancer cell lines. J Nucl Med 2008;49:1472–9.
Elsässer-Beile U, Reischl G, Wiehr S, Bühler P, Wolf P, Alt K, et al. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J Nucl Med 2009;50:606–11.
Ramli M, Smith SV, Lindoy LF. Investigation of novel bis- and tris-tetraazamacrocycle for use in the copper-64 ((64)Cu) radiolabeling of antibodies with potential to increase the therapeutic index for drug targeting. Bioconjug Chem 2009;20:868–76.
Voss SD, Smith SV, DiBartolo N, McIntosh LJ, Cyr EM, Bonab AA, et al. Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates. Proc Natl Acad Sci U S A 2007;104:17489–93.
Philpott GW, Schwarz SW, Anderson CJ, Dehdashti F, Connett JM, Zinn KR, et al. RadioimmunoPET: detection of colorectal carcinoma with positron-emitting copper-64-labeled monoclonal antibody. J Nucl Med 1995;36:1818–24.
Boswell CA, Sun X, Niu W, Weismann GR, Wong EH, Rheingold AL, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem 2004;47:1465–74.
Chong HS, Mhaske S, Lin M, Bhuniya S, Song HA, Brechibel MW, et al. Novel synthetic ligands for targeted PET imaging and radiotherapy of copper. Bioorg Med Chem Lett 2007;17:6107–10.
Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, et al. [64Cu-NOTA-8-Aoc-BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci U S A 2007;104:12462–7.
Sprague JE, Peng Y, Fiamengo AL, Woodkin KS, Southwick EA, Weisman GR, et al. Synthesis, characterization and in vivo studies of Cu(II)-64-labeled cross-bridged tetraazamacrocycle-amide complexes as models of peptide conjugate imaging agents. J Med Chem 2007;50:2527–35.
Ferreira CL, Yapp DTT, Lamsa E, Gleave M, Bensimon C, Jurek P, et al. Evaluation of novel bifunctional chelates for the development of Cu-64-based radiopharmaceuticals. Nucl Med Biol 2008;35:875–82.
Di Bartolo NM, Sargeson AM, Donlevy TM, Smith SV. Synthesis of a new cage ligand, SarAr, and its complexation with selected transition metal ions for potential use in radioimaging. Dalton Trans 2001:2303–9.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82.
Cai W, Niu G, Chen X. Multimodality imaging of the HER-kinase axis in cancer. Eur J Nucl Med Mol Imaging 2008;35:186–208.
Constantini DL, Bateman K, McLarty K, Vallis KA, Reilly RM. Trastuzumab-resistant breast cancer cells remain sensitive to the auger electron-emitting radiotherapeutic agent 111In-NLS-trastuzumab and are radiosensitized by methotrexate. J Nucl Med 2008;49:1498–505.
Constantini DL, Chan C, Cai Z, Vallis KA, Reilly RM. (111)In-labeled trastuzumab (Herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med 2007;48:1357–68.
Crow DM, Williams L, Colcher D, Wong JYC, Raubitschek A, Shively JE. Combined radioimmunotherapy and chemotherapy of breast tumors with Y-90-labeled anti-Her2 and anti-CEA antibodies with taxol. Bioconjug Chem 2005;16:1117–25.
Djikers ECF, Kosterink JGW, Rademaker AP, Perk LR, van Dongen GA, Bart J, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med 2009;50:974–81.
McLarty K, Cornelissen B, Scollard DA, Done SJ, Chun K, Reilly RM. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur J Nucl Med Mol Imaging 2009;36:81–93.
Orlova A, Nilsson FY, Wikman M, Widström C, Ståhl S, Carlsson J, et al. Comparative in vivo evaluation of technetium and iodine labels on an anti-HER2 affibody for single-photon imaging of HER2 expression in tumors. J Nucl Med 2006;47:512–9.
Orlova A, Wållberg H, Stone-Elander S, Tolmachev V. On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of 124I-labeled affibody molecule and trastuzumab in murine xenograft model. J Nucl Med 2009;50:417–25.
Ren G, Zhang R, Liu Z, Webster JM, Miao Z, Gambhir SS, et al. A 2-helix small protein labeled with 68Ga for PET imaging of HER2 expression. J Nucl Med 2009;50:1492–9.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92.
Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol 2004;22:701–6.
Tolmachev V, Orlova A, Pehrson R, Galli J, Baastrup B, Andersson K, et al. Radionuclide therapy of HER2-positive microxenografts using a 177Lu-labeled HER2-specific Affibody molecule. Cancer Res 2007;67:2773–82.
Dragowska WH, Warburton C, Yapp DTT, Minchinton AI, Hu Y, Waterhouse DN, et al. Her2/neu overexpression increases the viable hypoxic cell population within solid tumors without causing changes in tumor vascularization. Mol Cancer Res 2004;2:606–19.
Wieholt C, Hsiao I, Lin K, Chung Y, Chen C, Yen T. Performance evaluation of small animal PET system using NEMA standards. J Nucl Med 2008;49:119P.
Acknowledgements
The authors wish to thank D. Masin, M. Oosley and D. Strutt for animal husbandry, M. Belanger for image analysis and T. Ruth of TRIUMF for use of facilities.
Conflicts of interest
Cara L. Ferreira and Corinne Bensimon are employed by MDS Nordion. Paul Jurek and Garry Kiefer are employed by Macrocyclics Inc. The other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira, C.L., Yapp, D.T.T., Crisp, S. et al. Comparison of bifunctional chelates for 64Cu antibody imaging. Eur J Nucl Med Mol Imaging 37, 2117–2126 (2010). https://doi.org/10.1007/s00259-010-1506-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1506-1