Abstract
Purpose
The clinical potential of striatal imaging with dopamine transporter (DAT) SPECT tracers is hampered by the limited capability to recover activity concentration ratios due to partial volume effects (PVE). We evaluated the accuracy of a least squares method that allows retrieval of activity in regions of interest directly from projections (LS-ROI).
Methods
An Alderson striatal phantom was filled with striatal to background ratios of 6:1, 9:1 and 28:1; the striatal and background ROIs were drawn on a coregistered X-ray CT of the phantom. The activity ratios of these ROIs were derived both with the LS-ROI method and with conventional SPECT EM reconstruction (EM-SPECT). Moreover, the two methods were compared in seven patients with motor symptoms who were examined with N-3-fluoropropyl-2-β-carboxymethoxy-3-β-(4-iodophenyl) (FP-CIT) SPECT, calculating the binding potential (BP).
Results
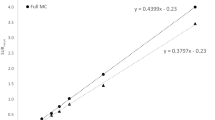
In the phantom study, the activity ratios obtained with EM-SPECT were 3.5, 5.3 and 17.0, respectively, whereas the LS-ROI method resulted in ratios of 6.2, 9.0 and 27.3, respectively. With the LS-ROI method, the BP in the seven patients was approximately 60% higher than with EM-SPECT; a linear correlation between the LS-ROI and the EM estimates was found (r = 0.98, p = 0.03).
Conclusion
The LS-ROI PVE correction capability is mainly due to the fact that the ill-conditioning of the LS-ROI approach is lower than that of the EM-SPECT one. The LS-ROI seems to be feasible and accurate in the examination of the dopaminergic system. This approach can be fruitful in monitoring of disease progression and in clinical trials of dopaminergic drugs.




Similar content being viewed by others
References
Innis RB, Seybil JB, Scanley BE, Laruelle M, Abi-Dargham A, Wallace E, et al. Single photon computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci U S A 1993;90(24):11965–69.
Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J Nucl Med 1998;39(11):1879–84.
Mozley PD, Schneider JS, Acton PD, Plossl K, Stern MB, Siderowf A, et al. Binding of [99mTc]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J Nucl Med 2000;41(4):584–9.
Acton PD, Newberg A, Plossl K, Mozley PD. Comparison of region-of-interest analysis and human observers in the diagnosis of Parkinson’s disease using [99mTc]TRODAT-1 and SPECT. Phys Med Biol 2006;51(3):575–85.
Soret M, Koulibaly PM, Darcourt J, Hapdey S, Buvat I. Quantitative accuracy of dopaminergic neurotransmission imaging with 123I SPECT. J Nucl Med 2003;44(7):1184–93.
Hashimoto J, Sasaki T, Ogawa K, Kubo A, Motomura N, Ichihara T, et al. Effects of scatter and attenuation correction on quantitative analysis of β-CIT brain SPET. Nucl Med Commun 1999;20(2):159–65.
Kojima A, Matsumoto M, Takahashi M, Hirota Y, Yashida H. Effect of spatial resolution on SPECT quantification values. J Nucl Med 1989;30(4):508–14.
Clarke LP, Leong LL, Serafini AN, Tyson IB, Silbiger ML. Quantitative SPECT imaging: influence of object size. Nucl Med Commun 1986;7(5):363–72.
Jaszczak JR, Coleman RE, Withehead FR. Physical factors affecting quantitative measurement using camera based single photon emission computed tomography (SPECT). IEEE Trans Nucl Sci 1981;28:69–80.
Koch W, Radau PE, Munzing W, Tatsch K. Cross-camera comparison of SPECT measurements of a 3-D antropomorphic basal ganglia phantom. Eur J Nucl Med Mol Imag 2006;33(4):495–502.
Chaly T, Dhawan V, Kazumata K, Antonini A, Margouleff C, Dahl JR, et al. Radiosynthesis of [18F]N-3-fluoropropyl-2β-carbomethoxy-3-β-(4-iodophenyl) nortropane and the first human study with positron emission tomography. Nucl Med Biol 1996;23:999–1004.
Kazumata K, Dhawan V, Chaly T, Antonini A, Margouleff C, Belakhlef A, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med 1998;39(9):1521–30.
Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998;39(5):904–11.
Cooke BE, Evans AC. A phantom to assess quantitative recovery of positron tomographs. J Comput Assist Tomogr 1983;7(5):876–80.
Mullani NA. A phantom for quantitation of partial volume effects in ECT. IEEE Trans Nucl Sci 1989;36(1):983–7.
Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr 1979;3(3):299–308.
Mahoney DK, Huang SC, Ricci AR, Mazziotta JC, Hoffman EJ, Phelps ME. A realistic computer simulated brain phantom for evaluation of PET characteristics. IEEE Trans Med Imag 1987;6:250–7.
Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr 1990;14(4):561–70.
Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12(4):571–83.
Meltzer CC, Zubieta JK, Links JM, Brakeman P, Stumpf MJ, Frost JJ. MR-based correction of brain PET measurements for heterogeneous gray matter radioactivity distribution. J Cereb Blood Flow Metab 1996;16(4):650–8.
Frouin V, Comtat C, Reilhac A, Gregoire MC. Correction for partial volume effect for PET striatal imaging: fast implementation and study of robustness. J Nucl Med 2002;43(12):1715–26.
Quarantelli M, Berkouk K, Prinster A, Landeau B, Svarer C, Balkay L, et al. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med 2004;45(2):192–201.
Du Y, Tsui BMW, Frey EC. Partial volume effect compensation for quantitative brain SPECT imaging. IEEE Trans Med Imag 2005;24(8):969–76.
Soret M, Koulibaly PM, Darcourt J, Buvat I. Partial volume effect correction in SPECT for striatal uptake measurements in patients with neurodegenerative diseases: impact upon patient classification. Eur J Nucl Med Mol Imag 2006;33(9):1062–72.
Formiconi AR. Least squares algorithm for region of interest evaluation in emission tomography. IEEE Trans Med Imag 1993;12(1):90–100.
Vanzi E, Formiconi AR, Bindi D, La Cava G, Pupi A. Kinetic parameter estimation from renal measurements with a three-headed SPECT system: a simulation study. IEEE Trans Med Imag 2004;23(3):363–73.
Huesman RH. A new fast algorithm for the evaluation of regions of interest and statistical uncertainty in computed tomography. Phys Med Biol 1984;29(5):543–52.
Muzic RF, Chen CH, Nelson AD. A method to correct for scatter, spillover, and partial volume effects in region of interest analysis in PET. IEEE Trans Med Imag 1998;17(2):202–13.
Chen CH, Muzic RF, Nelson AD, Adler LP. Simultaneous recovery of size and radioactivity concentration of small spheroids with PET data. J Nucl Med 1999;40(1):118–30.
Carson RE. A maximum likelihood method for region-of-interest evaluation in emission tomography. J Comput Assist Tomograph 1986;10(4):654–63.
Da Silva AJ, Tang HR, Wong KH, Wu MC, Dae MW, Hasegawa BH. Absolute quantification of regional myocardial uptake of 99mTc-sestamibi with SPECT: experimental validation in a porcine model. J Nucl Med 2001;42(5):772–9.
Formiconi AR, Passeri A, Calvini P. Theoretical determination of the collimator geometrical transfer function for the reconstruction of SPECT data. IEEE Trans Nucl Sci 1999;46(4):1075–80.
Boccacci P, Bonetto P, Calvini P, Formiconi AR. A simple model for the efficient correction of collimator blur in 3D SPECT imaging. Inverse Problems 1999;15:907–30.
Minoshima S, Koeppe RA, Frey KA, Kuhl D. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 1994;35(9):1528–37.
Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000;12(4):191–200.
Gelb D, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56(1):33–9.
Bertero M, Boccacci P. Introduction to inverse problems in imaging. Bristol and Philadelphia: Institute of Physics Publishing; 1998.
Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med Mol Imag 2000;27(7):867–9.
Tsuchida T, Ballinger JR, Vines D, Kim YJ, Utsunomiya K, Lang AE, et al. Reproducibility of dopamine transporter density measured with 123I-FPCIT SPECT in normal control and Parkinson’s disease patients. Ann Nucl Med 2004;18(7):609–16.
Du Y, Tsui BMW, Frey EC. Model-based compensation for quantitative 123I brain SPECT imaging. Phys Med Biol 2006;51(5):1269–82.
Kim KM, Varrone A, Watabe H, Shidahara M, Fujita M, Innis RB, et al. Contribution of scatter and attenuation compensation to SPECT images of nonuniformly distributed brain activities. J Nucl Med 2003;44(4):512–9.
Cot A, Falcon C, Crespo C, Sempau J, Pareto D, Bullich S, et al. Absolute quantification in dopaminergic neurotransmission SPECT using a Monte Carlo-based scatter correction and fully 3-dimensional reconstruction. J Nucl Med 2005;46(9):1497–504.
Meyer PT, Sattler B, Lincke T, Seese A, Sabri O. Investigating dopaminergic neurotransmission with 123I-FP-CIT SPECT: comparability of modern SPECT systems. J Nucl Med 2003;44(5):839–45.
Lorberboym M, Djaldetti R, Melamed E, Sadeh M, Lampl Y. 123I-FP-CIT SPECT imaging of dopamine transporters in patients with cerebrovascular disease and clinical diagnosis of vascular parkinsonism. J Nucl Med 2004;45(10):1688–93.
Acknowledgement
The authors would like to thank GE Healthcare for their collaboration in the performance of the Alderson phantom study.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
In the LS-ROI method, the generalised inverse is considered for the determination of a set of ROI values from raw tomographic data. The generalised inverse gives the least squares solution to a linear system of equations.
Actually, the tomographic problem can be described using a system of linear equations:
where p km is the measured projection at the m-th angle (m=1, .., M), bin k (k=1,.., K); Y ij is the number of emitted photons in the object pixel (i, j) and the elements \(F^{{km}}_{{ij}} \) describe the acquisition process as well as the geometrical system response (resolution, attenuation and scatter). By using a matrix notation, if we call Y the [IJ×1] object voxel concentration vector, p the [KM×1] projection data set and, finally, F the [KM×IJ] projection matrix, the previous equation can be rewritten as:
Least squares methods seek the minimisation of the functional
where σ km is the uncertainty with which p km is measured. The solution is given by:
where \(\Phi ^{{ - 1}}_{p} \) is the inverse of the projection covariance matrix. Φ p is a diagonal matrix whose diagonal elements are the variances of the projection data or, if an unweighted fit is performed, Φ p =I.
Equation 7 gives the standard least squares solution to Eq. 4. Application of Eq. 7 to the reconstruction of the tomographic images would be appealing because the solution could be obtained directly, without any iterative procedure, allowing the incorporation of a model of non-stationary factors. Unfortunately, Eq. 7 is extremely ill-conditioned, thus threatening the chance of finding an acceptable physical solution.
Let us suppose that our image space can be divided into a small number N R of ROIs with constant content, such that Y ij =X α for all pixels (i, j) belonging to the region α (R α), where α=1, ..,N R . Equation 4, therefore, becomes:
where \(G^{{km}}_{\alpha } \) are called “sinograms” of the regions and represent the projection along the ray at bin k, angle m, of an object which is equal to 1 for voxels belonging to region α and 0 elsewhere.
If we call X the [N R ×1] region concentration vector and G the [KM×N R ] region projection matrix, the (unweighted) least squares solution of Eq. 8 is:
Since we have introduced a lot of a priori information about the solution (which is constant over the ROIs), the ill-conditioning of the “ROI” problem (Eq. 9) is strongly reduced with respect to the original “voxel” problem (Eq. 7). Therefore, the solution can be computed directly from projections by using Eq. 9, once regions have been segmented in some way and the sinograms \(G^{{km}}_{\alpha } \) for each region have been obtained from the weighting factors \(F^{{km}}_{{ij}} \). Therefore, region activity evaluation is not performed with reconstructed images.
The covariance matrix of the solution can also be computed and is given by:
Rights and permissions
About this article
Cite this article
Vanzi, E., De Cristofaro, M.T., Ramat, S. et al. A direct ROI quantification method for inherent PVE correction: accuracy assessment in striatal SPECT measurements. Eur J Nucl Med Mol Imaging 34, 1480–1489 (2007). https://doi.org/10.1007/s00259-007-0404-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0404-7