Abstract
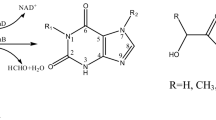
By polymerase chain reaction mutagenesis techniques, an NdeI restriction site was introduced at the initiation codon of the mannitol dehydrogenase (MDH) gene (mtlK) of Rhodobacter sphaeroides Si4. The mtlK gene was then subcloned from plasmid pAK74 into the NdeI site of the overexpression vector pET24a+ to give plasmid pASFG1. Plasmid pASFG1 was introduced into Escherichia coli BL21(DE3), which was grown in a 1.5-l bioreactor at 37 °C and pH 7.0. Overexpression of MDH in Escherichia coli BL21(DE3) [pASFG1] was determined by enzymatic analysis and sodium dodecyl sulfate (SDS)/polyacrylamide gel electrophoresis. Under standard growth conditions, E. coli produced considerable amounts of a polypeptide that correlated with MDH in SDS gels, but the activity yield was low. Decreasing the growth temperature to 27 °C and omitting pH regulation resulted in a significant increase in the formation of soluble and enzymatically active MDH up to a specific activity of 12.4 U/mg protein and a yield of 26 000 U/l, which corresponds to 0.38 g/l MDH. This was an 87-fold overexpression of MDH compared to that of the natural host R. sphaeroides Si4, and a 236-fold improvement of the volumetric yield. MDH was purified from E. coli BL21(DE3) [pASFG1] with 67% recovery, using ammo-nium sulfate precipitation, hydrophobic interaction chromatography, and gel filtration. Partial characterization of the recombinant MDH revealed no significant differences to the wild-type enzyme.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 18 February 1997 / Received revision: 27 March 1997 / Accepted: 27 March 1997
Rights and permissions
About this article
Cite this article
Schäfer, A., Stein, M., Schneider, KH. et al. Mannitol dehydrogenase from Rhodobacter sphaeroides Si4: subcloning, overexpression in Escherichia coli and characterization of the recombinant enzyme. Appl Microbiol Biotechnol 48, 47–52 (1997). https://doi.org/10.1007/s002530051013
Issue Date:
DOI: https://doi.org/10.1007/s002530051013