Abstract
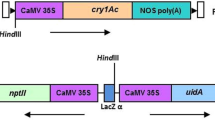
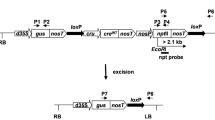
The most crucial yield constraint of pigeon pea is susceptibility to the pod borer Helicoverpa armigera, which causes extensive damage and severe economic losses every year. The Agrobacterium-mediated plumular meristem transformation technique was applied for the development of cry1Ac transgenic pigeon pea. Bioactivity of the cry1Ac gene was compared based on integration and expression driven by two promoters, the constitutive CaMV35S promoter and the green-tissue-specific ats1A promoter, in those transgenic events. The transgenic events also contained the selectable marker gene nptII flanked by loxP sites. Independent transgenic events expressing the Cre recombinase gene along with a linked bar selection marker were also developed. Integration and expression patterns of both cry1Ac and cre were confirmed through Southern and western blot analysis of T1 events. The constitutive expression of the Cry1Ac protein was found to be more effective for conferring resistant activity against H. armigera larvae in comparison to green-tissue-specific expression. Constitutively expressing Cry1Ac T1 events were crossed with Cre recombinase expressing T1 events. The crossing-based Cre/lox-mediated marker gene elimination strategy was demonstrated to generate nptII-free Cry1Ac-expressing T2 events. These events were subsequently analyzed in the T3 generation for the segregation of cre and bar genes. Five Cry1Ac-expressing T3 transgenic pigeon pea events were devoid of the nptII marker as well as cre-bar genes. H. armigera larval mortality in those marker-free T3 events was found to be 80–100%. The development of such nptII selectable marker-free Cry1Ac-expressing pigeon pea transgenics for the first time would greatly support the sustainable biotechnological breeding program for pod borer resistance in pigeon pea.
Key points
• Constitutive expression of Cry1Ac conferred complete resistance against Helicoverpa armigera
• Green-tissue-specific expression of Cry1Ac conferred partial pest resistance
• Cre/lox-mediated nptII elimination was successful in constitutively expressing Cry1Ac transgenic pigeon pea events.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its Supplementary Information files].
References
Acharjee S, Sarmah BK, Kumar PA, Olsen K, Mahon R, Moar WJ, Moore A, Higgins TJ (2010) Transgenic chickpeas (Cicer arietinum L) expressing a sequence-modified cry2Aa gene. Plant Sci 178(3):333–339
Bala A, Roy A, Das A, Chakraborti D, Das S (2013) Development of selectable marker free, insect resistant, transgenic mustard (Brassica juncea) plants using Cre/lox mediate recombination. BMC Biotechnol 13(1):88
Bakhsh A, Qayyum RA, Shamim Z, Husnain T (2011) A mini review: RuBisCo small subunit as a strong, green tissue-specific promoter. Arch Biol Sci 63(2):299–307
Bayley CC, Morgan M, Dale EC, Ow DW (1992) Exchange of gene activity in transgenic plants catalyzed by the Cre-lox site-specific recombination system. Plant Mol Biol 18:353–362
Bradford MM (1976) A rapid and sensitive method for the quantitation of proteins using the principle of protein dye binding. Anal Biochem 72:248–254
Chakraborti D, Sarkar A, Gupta S, Das S (2006) Small and large scale genomic DNA isolation protocol for chickpea (Cicer arietinum L) suitable for molecular marker and transgenic analyses. Afr J Biotechnol 5(8):585–589
Chakraborti D, Sarkar A, Mondal H, Schuermann D, Hohn B, Sarmah B, Das S (2008) Cre/lox system to develop selectable marker free transgenic tobacco plants conferring resistance against sap sucking hemipteran insect. Plant Cell Rep 27:1623–1633
Chanda Venkata SK, Nadigatla Veera Prabha Rama GR, Saxena RK, Saxena K, Upadhyaya HD, Siambi M, Silim SN, Reddy KN, Hingane AJ, Sharma M, Sharma S (2019) Pigeonpea improvement: an amalgam of breeding and genomic research. Plant Breed 138(4):445–454
Choudhary AK, Raje RS, Datta S, Sultana R, Ontagodi T (2013) Conventional and molecular approaches towards genetic improvement in pigeonpea for insect resistance. Amer J Plant Sci 4:372–385
Dale EC, Ow DW (1991) Gene transfer with subsequent removal of the selection gene from the host genome. P Natl Acad Sci USA 88:10558–10562
Das A, Datta S, Sujayanand GK, Kumar M, Singh AK, Shukla A, Ansari J, Faruqui L, Thakur S, Kumar PA, Singh NP (2016) Expression of chimeric Bt gene, Cry1Aabc in transgenic pigeonpea (cv Asha) confers resistance to gram pod borer Helicoverpa armigera Hubner. Plant Cell Tissue Organ Cult 127(3):705–15
FAO (2019) Food and Agricultural Organization of the United Nation, FAO Statistical Database. http://faostat.fao.org. Accessed 1st May 2021
Ganguly S, Ghosh G, Purohit A, Sreevathsa R, Chaudhuri RK, Chakraborti D (2017) Effective screening of transgenic pigeonpea in presence of negative selection agents. Proc Natl Acad Sci India Sect B Biol Sci 88(4):1565–1571
Ganguly S, Ghosh G, Purohit A, Chaudhuri RK, Chakraborti D (2018) Development of transgenic pigeonpea using high throughput plumular meristem transformation method. Plant Cell Tissue Organ Cult 135(1):73–83
Ghosh G, Purohit A, Ganguly S, Chaudhuri RK, Chakraborti D (2014) In vitro shoot grafting on rootstock: an effective tool for Agrobacterium-mediated transformation of pigeonpea (Cajanus cajan (L.) Millsp.). Plant Biotechnol 31:301–308
Ghosh G, Ganguly S, Purohit A, Chaudhuri RK, Das S, Chakraborti D (2017) Transgenic pigeonpea events expressing Cry1Ac and Cry2Aa exhibit resistance to Helicoverpa armigera. Plant Cell Rep 36(7):1037–1051
Hazarika N, Acharjee S, Boruah RR, Baba K, Parimi S, Char B, Armstrong J, Moore A, Higgins TJ, Sarmah BK (2019) Enhanced expression of Arabidopsis rubisco small subunit gene promoter regulated Cry1Ac gene in chickpea conferred complete resistance to Helicoverpa armigera. J Plant Biochem Biotechnol 1–11
Hoa TTC, Bong BB, Huq E, Hodge TK (2002) Cre/lox site specific recombination controls the excision of a transgene from the rice genome. Theor Appl Genet 104:518–525
Jaiwal PK, Sahoo L, Singh ND, Singh RP (2002) Strategies to deal with the concern about marker genes in transgenic plants: some environment-friendly approaches. Curr Sci 83(2):128–136
Kaur A, Sharma M, Sharma C, Kaur H, Kaur N, Sharma S, Arora R, Singh I, Sandhu JS (2016) Pod borer resistant transgenic pigeon pea (Cajanus cajan L) expressing cry1Ac transgene generated through simplified Agrobacterium transformation of pricked embryo axes. Plant Cell Tissue Organ Cult 127(3):717–727
Kerbach S, Lorz H, Becker D (2005) Site-specific recombination in Zea mays. Theor Appl Genet 111:1608–1616
Konig A (2003) A framework for designing transgenic crops—science, safety and citizen’s concerns. Nat Biotechnol 21(11):1274–1279
Kopertekh L, Jüttner G, Schiemann J (2004) PVX-Cre-mediated marker gene elimination from transgenic plants. Plant Mol Biol 55(4):491–500
Krishna G, Reddy PS, Ramteke PW, Rambabu P, Tawar KB, Bhattacharya P (2011) Agrobacterium-mediated genetic transformation of pigeon pea [Cajanus cajan (L) Millsp] for resistance to legume pod borer Helicoverpa armigera. J Crop Sci Biotechnol 14(3):197–204
Kumar S, Timko MP (2004) Enhanced tissue-specific expression of the herbicide resistance bar gene in transgenic cotton (Gossypium hirsutum L cv Coker 310FR) using the Arabidopsis rbcS ats1A promoter. Plant Biotechnol J 21(4):251–259
Kumar S, Chandra A, Pandey KC (2008) Bacillus thuringiensis (Bt) transgenic crop: an environment friendly insect-pest management strategy. J Environ Biol 29(5):641–653
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation–competent Arabidopsis genomic library in Agrobacterium. Bio/technol 9(10):963–7
Miki B, McHugh S (2004) Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotechnol 107(3):193–232
Miklos JA, Alibhai MF, Bledig SA, Connor-Ward DC, Gao AG, Holmes BA, Kolacz KH, Kabuye VT, MacRae TC, Paradise MS, Toedebusch AS (2007) Characterization of soybean exhibiting high expression of a synthetic Bacillus thuringiensis cry1A transgene that confers a high degree of resistance to lepidopteran pests. Crop Sci 47(1):148–157
Mishra RK, Bohra A, Kamaal N, Kumar K, Gandhi K, Sujayanand GK, Saabale PR, SN SJ, Sarma BK, Kumar D, Mishra M (2018) Utilization of biopesticides as sustainable solutions for management of pests in legume crops: achievements and prospects. Egypt J Biol Pest Co 28(1):3
Mondal HA, Chakraborti D, Majumder P, Roy P, Roy A, Gupta Bhattacharya S, Das S (2011) Allergenicity assessment of Allium sativum leaf agglutinin (ASAL) a potential candidate protein for developing sap sucking insect resistant food crops. PLoS One 6(11):e27716
Muzaffar A, Kiani S, Khan MAU, Rao AQ, Ali A, Awan MF, Iqbal A, Nasir IA, Shahid AA, Husnain T (2015) Chloroplast localization of Cry1Ac and Cry2A protein-an alternative way of insect control in cotton. Biol Res 48(1):1–11
Perlak FJ, Deaton RW, Armstrong TA, Fuchs RL, Sims SR, Greenplate JT, Fischhof DA (1990) Insect resistant cotton plants. Bio/technology 8:939–943
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev Biol 40(1):1–22
Ramessar K, Peremarti A, Go´mez-Galera S, Naqvi S, Moralejo M, Munoz P, Capell T, Christou P (2007) Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants: a case of the science not supporting the politics. Transgenic Res 16:261–280
Ramu SV, Rohini S, Keshavareddy G, Neelima MG, Shanmugam NB, Kumar ARV, Sarangi SK, Ananda Kumar P, Udayakumar M (2012) Expression of a synthetic cry1AcF gene in transgenic pigeonpea confers resistance to Helicoverpa armigera. J Appl Entomol 136:675–687
Sarkar S, Roy S, Ghosh SK (2021) Development of marker-free transgenic pigeon pea (Cajanus cajan) expressing a pod borer insecticidal protein. Sci Rep 11:10543
Saxena RK, Saxena KB, Pazhamala LT, Patel K, Parupalli S, Sameerkumar CV, Varshney RK (2015) Genomics for greater efficiency in pigeonpea hybrid breeding. Front Plant Sci 6:793
Schenk ST, Schikora A (2015) Staining of callose depositions in root and leaf tissues. Bio-Protoc 5(6):1429
Sengupta S, Chakraborti D, Mondal HA, Das S (2010) Selectable antibiotic resistance marker gene-free transgenic rice harbouring the garlic leaf lectin gene exhibits resistance to sap-sucking planthoppers. Plant Cell Rep 29(3):261–271
Sharma KK, Lavanya K, Anjaiah A (2006) Agrobacterium tumefaciens-mediated production of transgenic pigeonpea (Cajanus cajan L. Millsp.) expressing the synthetic Bt Cry1AB gene. In Vitro Cell Dev Biol Plant 42:165–173
Sharma HC, Sujana G, Rao DM (2009) Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeonpea. Arthropod Plant Interact 3(3):151–161
Singh S, Kumar NR, Maniraj R, Lakshmikanth R, Rao KY, Muralimohan N, Arulprakash T, Karthik K, Shashibhushan NB, Vinutha T, Pattanayak D (2018) Expression of Cry2Aa, a Bacillus thuringiensis insecticidal protein in transgenic pigeonpea confers resistance to gram pod borer. Helicoverpa Armigera Sci Rep 8(1):1–2
Sreekala C, Wu L, Gu K, Wang D, Tian D, Yin Z (2005) Excision of a selectable marker in transgenic rice (Oryza sativa L.) using a chemically regulated Cre /loxP system. Plant Cell Rep 24:86–89
Sreekanth M, Lakshmi MSM, Rao YK (2014) Bio-efficacy and economics of certain new insecticides against gram pod borer Helicoverpa armigera (Hubner) infesting pigeonpea (Cajanus cajan L). Int J Plant Animal Env Sci 4(1):11–15
Srivastava V, Ow DW (2003) Rare instances of Cre-mediated deletion product maintained in transgenic wheat. Plant Mol Biol 52:661–668
Surekha C, Beena MR, Arundhati A, Singh PK, Tuli R, Dutta-Gupta A, Kirti PB (2005) Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci 169:1074–1080
Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A, Higgins TJ (1995) A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci 73(9):2752–2759
Thu TT, Dewaele E, Trung LQ, Claeys M, Jacobs M, Angenon G (2007) Increasing lysine levels in pigeonpea (Cajanus cajan (L) Millsp) seeds through genetic engineering. Plant Cell Tissue Organ Cult 91:35–143
Upadhyaya CP, Nookaraju A, Gururani MA, Upadhyaya DC, Kim DH, Chun SC, Park SW (2010) An update on the progress towards the development of marker-free transgenic plants. Bot Stud 51(3):277–292
Varshney RK, Penmetsa RV, Dutta S, Kulwal PL, Saxena RK, Datta S, Sharma TR, Rosen B, Carrasquilla-Garcia N, Farmer AD, Dubey A (2010) Pigeonpea genomics initiative PGI an international effort to improve crop productivity of pigeonpea (Cajanus cajan L). Mol Breed 26(3):393–408
Varshney RK, Chen W, Li Y, Bharti AK, Saxena RK, Schlueter JA (2012) Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nat Biotechnol 30:83–89
Wong EY, Hironaka CM, Fischhoff D (1992) Arabidopsis thaliana small subunit leader and transit peptide enhance the expression of Bacillus thuringiensis proteins in transgenic plants. Plant Mol Biol 20(1):81–93
Yau YY, Stewart CN (2013) Less is more: strategies to remove marker genes from transgenic plants. BMC Biotechnol 13(1):36
Zhang W, Subbarao S, Addae P, Shen A, Armstrong C, Peschke V, Gilbertson L (2003) Cre/lox-mediated marker gene excision in transgenic maize (Zea mays L.) plants. Theor Appl Genet 107:1157–1168
Acknowledgements
The authors acknowledge the Indian Council of Agricultural Research for the financial support (grant number NFBSFARA/PB2010/2010-11); DST-PURSE, University of Calcutta, St. Xavier’s College, and Bose Institute, Kolkata, India, for the infrastructure support. S. Ganguly A. Purohit and S. Ghosh thank West Bengal Higher Education Department-Swami Vivekananda Merit cum Means Scholarship (No. 52-Edn (B) l 5B-l s/2017), Council of Scientific and Industrial Research, India (File No. 08/548(0007)/2018 EMR-I), and the Department of Science and Technology, Govt. of India, INSPIRE fellowship (DST/INSPIRE Fellowship/2018/IF180158), respectively, for providing the fellowship.
Funding
Indian Council of Agricultural Research,NFBSFARA/PB2010/2010-11,Dipankar Chakraborti
Author information
Authors and Affiliations
Contributions
SG, AP, SG, and DC conceived and designed all of the experiments. SG, AP, and SG conducted all the experiments. RKC, SD, and DC were responsible for the data analysis and supervision of the work. SG and DC drafted and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants. The authors did not perform any animal-based experiments for this work.
Consent for publication
Review work is presented in this manuscript with the consent of all authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ganguly, S., Purohit, A., Ghosh, S. et al. Clean gene technology to develop selectable marker-free pod borer-resistant transgenic pigeon pea events involving the constitutive expression of Cry1Ac. Appl Microbiol Biotechnol 106, 3051–3067 (2022). https://doi.org/10.1007/s00253-022-11922-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11922-1