Abstract
Bacopa monnieri (L.) Wettst. (BM), also known as ‘Brahmi’ or ‘Water Hyssop’, has been utilized as a brain tonic, memory enhancer, sensory organ revitalizer, cardiotonic, anti-anxiety, antidepressant and anticonvulsant agent in the Indian system of medicine Ayurveda for centuries. BM is beneficial in the treatment of Parkinson’s disease, Alzheimer’s disease, epileptic seizures and schizophrenia in recent pharmacological research. Dammarane-type triterpenoid saponins containing jujubogenin and pseudojujubogenin as aglycones, also known as bacosides, are the principal chemical ingredients identified and described from BM. Bacosides have been shown to have anti-ageing, anticancer, anticonvulsant, antidepressant, anti-emetic, anti-inflammatory and antibacterial properties in a variety of pre-clinical and clinical studies. The pharmaceutical industry’s raw material comes from wild sources; nevertheless, the concentration of bacosides varies in different regions of the plants, as well as seasonal and genotypic variation. Cell and tissue cultures are appealing alternatives for the long-term manufacture of bioactive chemicals, and attempts to produce bacosides using in vitro cultures have been made. This review discusses the biotechnological approaches used to produce bacosides, as well as the limitations and future potential.
Key points
• Bacosides extracted from Bacopa monnieri are important pharmaceutical compounds.
• The current review provides insight into biotechnological interventions for the production of bacosides using in vitro cultures.
• Highlights the prospects improvement of bacoside production through metabolic engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The medicinal plant Bacopa monnieri (L.) Wettst. (BM), which belongs to the Scrophulariaceae family (currently in the Plantaginaceae family), is also known as ‘Brahmi’, ‘Jalabrahmi’ or ‘Water Hyssop’ (National Medicinal Plant Board (NMPB) 2021). It is a perennial herbaceous plant with simple, sessile fleshy leaves that grows prostrately. Flowers are axillary, single and whitish or pale blue (Fig. 1). BM thrives in wetland environments near streams and lake beds and is found in India, Pakistan, Afghanistan, Nepal, Sri Lanka, North and South America, tropical Asia, Africa and Australia’s plains (National Medicinal Plant Board (NMPB) 2021). For millennia, BM has been utilized in the Indian medicine Ayurveda for the treatment of anxiety and the improvement of cognition and memory. BM is also beneficial in the treatment of Parkinson’s disease, Alzheimer’s disease, epilepsy and schizophrenia (Shalini et al. 2021). Furthermore, anti-ageing, anticancer, anticonvulsant, antidepressant, anti-emetic, anti-inflammatory and antibacterial properties have been identified for BM (Banerjee et al. 2021).
Dammarane triterpenoid glycosides, sterols, sterol glycosides, phenylethanoid glycosides, cucurbitacins and alkaloids are among the secondary metabolites produced by BM. Among these secondary metabolites, triterpene saponins of the dammarane class, also known as bacosides and bacosaponins, are important constituents of BM and are responsible for several pharmacological actions. Jujubogenin and pseudojujubogenin are saponins that differ only in the number of sugar units in the glycosidic chain and the position of the olefinic side chain in the aglycone (Figs. 2 and 3). Chatterji et al. (1965) were the first to isolate bacoside A (3-(-l-arbinopyranosyl)-O-β-d-glucopyranoside-10,20-dihydroxy-16-keto-dammar-24-ene). Bacoside A coexists with bacoside B, which differs only in optical rotation, according to later reports. Bacoside A and bacoside B were later discovered to be a combination of four triglycosidic saponins and four diglycosidic saponins, respectively (Deepak et al. 2005). Bacoside A has a chemical composition of bacoside A3, bacopaside II, bacopaside X and bacosaponin C (Deepak et al. 2005), whereas bacoside B has a chemical composition of bacopaside N1, bacopaside N2, bacopaside IV and bacopaside V. Bacosides A1 and A3; bacosaponins A, B, C, D, E, F, G, H and I; and bacopasides I, II, III, IV, V, VI, VII and VIII are other saponins reported from BM (Bhandari et al. 2020).
Clinical studies have shown that BM reduces the rate of memory loss and newly acquired information, improves cognitive performance, improves memory performance and is effective in the treatment of anhedonia (Micheli et al. 2020). In the food processing sector, BM is considered as a functional food ingredient because of its diverse medical value. There are several food products on the market that contain BM as a functional food ingredient, including beverages, energy drinks, syrups, granules and powders (Devendra et al. 2018). BM-based herbal medications rich in bacoside A, such as ‘Bacopa Plus’, ‘BacoMind’, ‘KeenMind’ and ‘Mentat’, are gaining popularity in many nations. Furthermore, BM is a key component of the traditional Indian bioactive health supplement ‘Chyawanprash’ (Sharma et al. 2019a). India’s annual BM usage is estimated to be 1000 tonnes. The majority of plant material utilized in the pharmaceutical and food industries comes from the wild. The continued use of BM from natural habitats has resulted in the native population being depleted. The National Medicinal Plants Board, Ministry of Science and Technology, Government of India, has identified BM as one of sixty medicinal plants that should be prioritized (National Medicinal Plant Board (NMPB) 2021). BM was also included in the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (National Medicinal Plant Board (NMPB) 2021). Recent studies have shown that bacoside content varies in different parts/organs of the BM plant, as well as accessions acquired from different regions and that bacoside content varies seasonally (Naik et al. 2012). Due to the aforementioned factors, additional ways for producing BM biomass with higher bacoside concentrations are required to meet the market’s increased demand. Plant cell and organ cultures are viable options for producing plant biomass as well as useful secondary metabolites. Suspension cultures of cells, adventitious roots, shoots and genetically modified hairy roots provide a consistent and stable source of natural goods (Espinosa-Leal et al. 2018; Gutierrez-Valdes et al. 2020; Murthy et al. 2021). Furthermore, by changing the chemical/physical parameters of the cultures and using bioprocess parameters in bioreactors, such cultures can be scaled up (Murthy et al. 2021). The potential for leveraging plant cell and tissue culture methods for the regulated production of bacosides is discussed in this paper. For improving bacoside accumulation in cell and organ cultures, various techniques such as elicitation, genetic transformation, metabolic engineering and bioreactor cultures are being addressed. This review provides researchers with important information to further develop and implement the massive production of bacosides by biotechnological approaches and methodologies.
Production of bacosides from cell suspension cultures of Bacopa monnieri
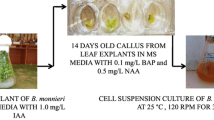
Rahman et al. (2002) established cell suspension cultures in BM using callus obtained from tissue-grown plant leaf explants. They also found that when cells were cultivated in Murashige and Skoog (MS) medium supplemented with 1 mg l−1 naphthalene acetic acid (NAA) and 0.5 mg l−1 kinetin (KIN) for 40 days, they were able to accumulate five–sixfold biomass. The BM cell biomass acquired from cell suspension culture was found to be capable of accumulating 10 mg g−1 dry weight (DW) bacoside A (Table 1). Bansal et al. (2017) used statistical design methodologies to undertake medium optimization experiments in shake flask cultures to increase biomass and bacoside A production. They conducted independent medium optimization trials to find the optimal sucrose or glucose (20 g l−1), KNO3/NH4NO3 ratio, NAA and KIN concentration, pH and MS medium concentration while holding all other variables constant. They employed the MS medium and 1 g l−1 fresh weight inoculum in 250-ml Erlenmeyer flasks containing 50 ml culture media to establish cultures. According to Bansal et al. (2017), glucose, KNO3 and KH2PO4 at concentrations of 5.67%, 0.313% and 0.29%, respectively, and an inoculum density of 0.66% in the baseline MS medium resulted in excellent cell growth (5.52 to 12.58 g l−1 fresh cell weight) and bacoside A synthesis (5.56 to 9.84 mg g−1 DW). BM cell suspension cultures were generated using the MS medium containing 0.1 mg l−1 6-benzylaminopurine (BAP) and 0.5 mg l−1 NAA by Leonard et al. (2018) with the goal of statistical optimization of BM cell cultures (Plackett–Burman statistical approach). The best concentration and critical variables were the inoculum size of 2 g l−1, sucrose concentration of 30 g l−1 and KH2PO4 concentration of 1.24 mM in one-sixth-strength MS medium, according to their findings. With these optimal conditions, they were able to achieve a maximum biomass production of 3.65 g l−1 and 0.49 mg g−1 DW of bacoside A. Seth et al. (2020) used a response surface approach in BM (variety CIM-Jagriti) cell suspension cultures to optimize bacoside A production. The effect of pH, photoperiod, NAA and BAP concentration on bacoside A biosynthesis was studied using a central composite design. They found that with a 5.4 pH, 18-h/6-h light/dark photoperiod and 1.2 mg l−1 BAP in combination with 0.2 mg l−1 NAA, they could get the optimum biomass concentration of 4.56 g l−1 DW and bacoside A production of 14.04 mg g−1 DW. Different elicitors, such as jasmonic acid (JA) and salicylic acid (SA) at 1 mg l−1, as well as additives, such as calcium pantothenate, cholesterol and sodium nitroprusside at 1 mg l−1, were tested on BM cell cultures by Koul and Mallubhotla (2020). They evaluated the effect of elicitors/additives on cell suspensions cultured in Gamborg’s (B5) liquid medium supplemented with 1 mg l−1 2,4-dichlorophenoxy acetic acid (2,4-D) for 3 days, 6 days, 9 days and 15 days. Salicylic acid induced the highest amount of bacoside accumulation of 6.58 mg g−1 DW of all the elicitors/additives examined. All of the preceding studies show that BM cell suspension cultures are capable of producing bacoside. Within vitro-grown cells, however, biomass and metabolite output are not substantial. It has long been known that secondary metabolite accumulation in plants is genotype and organ-specific (Naik et al. 2012). As a result, selecting superior genotypes for the establishment of cell suspension cultures is critical. Seth et al. (2020) used the BM variety CIM-Jagriti, which is a selection made by the Council of Scientific and Industrial Research-Regional Research Laboratory, Jammu, India, and is a superior variety in terms of biomass yield and bacoside content. Seth et al. (2020) did not, however, test a wide range of media for culture initiation. The important variables for cell growth and multiplication, as well as the accumulation of metabolites, are the choice of a suitable medium, optimal nutrient concentration and growth regulators (Murthy et al. 2021). Thus, with cell suspension cultures of BM, systematic procedures such as selection of superior genotype/plant, explant source, cell line selection and optimization of culture media, growth regulators and nutrition elements would have yielded better results.
Production of bacosides from shoot cultures of Bacopa monnieri
This section of the current review summarizes the present state of research on the generation of bacosides from BM shoot cultures. On the MS medium supplemented with benzyladenine (BA) or KIN, Shrivastava and Rajani (1999) regenerated adventitious shoots from BM leaf and stem explants. They discovered that the phytochemical profile of regenerated plants was identical to that of field-grown plants using qualitative thin-layer chromatographic analysis (Table 2). Praveen et al. (2009) used leaf explants to regenerate adventitious shoots of BM on the MS medium with KIN (2 mg l−1) and investigated the effects of liquid versus semi-solid medium on shoot regeneration. They demonstrated that liquid cultures produce more shoot biomass than semi-solid cultures. Explants grown in a liquid medium produced 155.6 shoots per explant, while explants grown in a semi-solid medium produced 64.4 shoots per explant. The amount of bacoside A in shoots regenerated in a liquid medium (11.92 mg g−1 DW) was 2.2-fold higher than that in shoots grown in semi-solid cultures. Glycine (0–125 μM), ferulic acid (0–200 μM), phenylalanine (0–200 μM), α-ketoglutaric acid (0–200 μM) and pyruvic acid (0–200 μM) were tested on the synthesis of bacoside A in BM shoot cultures by Parale et al. (2010). Among the organic supplements employed, 100 μM pyruvic acid significantly increased bacoside A production (35.2 mg g−1 DW), which was 3.8 times greater than that of the control plants (12.8 mg g−1 DW). The influence of macro-elements and the nitrogen source of MS medium on biomass accumulation and bacoside A production from BM adventitious shoot cultures were investigated by Naik et al. (2011). The leaf explants were grown in the MS medium supplemented with 2 mg l−1 KIN and various doses of NH4NO3, KNO3, calcium chloride (CaCl2), MgSO4, KH2PO4 and NH4+/NO3−. On the MS medium containing 2.0 × NH4NO3, they obtained the highest levels of shoot biomass (99.33 shoots and 0.15 g−1 DW) and bacoside A (17.92 mg g−1 DW). Muszynska et al. (2016) used shoot cultures of BM to investigate the effects of growth supplements such as magnesium sulphate (0.1 mg l−1), zinc hydroaspartate (0.1 mg l−1), l-tryptophan (0.1 mg l−1), serine (0.25 mg l−1 and 0.5 mg l−1) and anthranilic acid (0.5 mg l−1) on bacoside A accumulation. The addition of 0.1 mg l−1 anthranilic acid aided in the accumulation of bacoside, according to their findings (28.8 mg g−1 DW). As Jauhari et al. (2019) used 1 mg l−1 jasmonic acid, salicylic acid and malt extract on BM shoot cultures for 4 weeks, they found 3.9-, 3.2- and 2.7-fold increases in bacoside A accumulation when compared to non-elicited cultures. Dey et al. (2019) employed nodal explants on the MS medium with growth regulators BAP and thidiazuron (TDZ) at 0.5 mg l−1, 1 mg l−1, 1.5 mg l−1 and 2 mg l−1 to verify the role of polyamines such as spermine, spermidine and putrescine (0.5 mM and 1 mM) on BM plant regeneration and bacoside content. Polyamines promoted shoot regeneration, according to Dey et al. (2019), and regenerated shoots which acquired higher bacoside content (27.89 mg g−1 DW) than mother plants (25.46 mg g−1 DW). The above investigations demonstrate that shoot cultures are superior for the production of bacosides when compared to cell suspension cultures because shoots are organized structures and are responsible for a higher growth index (Table 2). It was well documented that the low yield of secondary metabolites in cell cultures is due to a lack of cellular differentiation. In contrast to this, organized structures like roots and shoots are attractive alternatives for the production of plant secondary metabolites (Murthy et al. 2021).
Production of bacosides from the genetically transformed shoot and root cultures of Bacopa monnieri
Majumdar et al. (2011) used the Agrobacterium rhizogenes LBA 9402 and A4 to genetically transform BM with aaM, iaaH and acs genes. They found that transformed roots induced by strain LBA 9402 were stable, but transformed roots induced by strain A4 dedifferentiated into a callus and spontaneously regenerated shoots (Table 3). Majumdar et al. (2011) analysed many bacosides in transformed shoots, including bacoside A3; bacosaponins C, D, E and F; and bacopasides II, III and V. Four saponins (bacosaponin D, bacosaponin F, bacopaside II and bacopaside V) were found to be fivefold greater in transformed plants than in non-transformed shoots. Majumdar et al. (2012) used the gene encoding cryptogein, a pertinacious elicitor, to carry out genetic transformation in BM utilizing A. rhizogenes LBA 9402 and A.tumefaciens strains containing crypt. They created Ri- and Ti-based crypt-transgenic BM plants and measured the levels of bacoside A3, bacosaponin C, bacosaponin D, bacopaside II, bacopaside III and bacopaside V in the transgenic plants. When compared to non-transformed plants, Ti crypt plants showed sixfold, sixfold and twofold increases in bacoside A3, bacosaponin D and bacopaside III, respectively. When comparing Ri crypt plants to Ri-transformed plants, they found fourfold, threefold, fourfold and twofold increases in bacopaside A3, bacopaside II, bacopaside III and bacopaside V levels, respectively. Paul et al. (2015) used A. rhizogenes and A. tumefaciens to genetically modify BM, raising transformed BM lines and maintaining them in vitro for 4 years. In vitro, the crypt-transformed lines maintained under long-term culture produced much more bacoside (1.66 to 2.05 times) than non-transformed plants. Largia et al. (2016) used leaf explants to transform BM with the A. rhizogenes strains A4, MTCC 532, MTCC 2364 and R 1000. Hairy roots from A4 and MTCC 532 strains showed shoot regeneration on the MS medium supplemented with appropriate phytohormones, out of four strains examined. For 2 weeks, hairy root–developed shoots were cultivated in a liquid medium supplemented with 10 mg l−1 chitosan as an elicitor. When compared to modified plants without elicitation, the genetically transformed plants produced 5.83% bacoside A, which is four times greater.
Bansal et al. (2014) used A. rhizogenes strains A4, R 1000, SA79, MTCC 532 and MTCC 2364 to generate hairy roots in BM. The line produced by strain MTCC 2364 had the highest biomass accumulation (6.8 g l−1 fresh biomass) and bacoside A concentration (10.02 mg g−1 DW) of the different hairy root lines (Table 3). Bansal et al. (2015) used response surface methodology to optimize MS medium components for the cultivation of hairy roots, and the optimized medium components were 41.72 g l−1 glucose, 6.12 g l−1 KNO3, 0.33 g l−1 KH2PO4 and 0.53 g l−1 MgSO4·7H2O. Bansal et al. (2015) used optimized settings to obtain optimal biomass (6.8 to 12.99 g l−1 fresh biomass) and bacoside A concentration (10.02 to 16.14 mg g−1 DW). According to the studies reported in the preceding section, shoot cultures are amenable to culturing and can acquire larger biomass and bacoside content. Furthermore, plants/shoots that have been genetically modified with the cryptogein gene may be able to accumulate a considerable amount of bacosides. Several researchers also developed stable hairy roots that might participate in the growth and accumulation of biomass and bacosides. Stable, promising shoot and hairy root lines should be preserved and used for bioreactor scale-up cultures.
Production of bacosides from shoot cultures of Bacopa monnieri in bioreactors
Sharma et al. (2015) used MS liquid medium supplemented with 1 mg l−1 BAP to evaluate the production of BM shoot biomass and bacosides in bioreactors such as Growtek (1 l capacity) and modified benchtop airlift bioreactors (5 l capacity) and compared them to shake flasks (1 l capacity). After 4 weeks of incubation, they found that airlift bioreactors (5.84), Growtek bioreactors (4.22) and shaking flasks (2.61) had the highest growth index (in terms of dry weight). Furthermore, in airlift bioreactors, Growtek bioreactors and shake flasks, bacoside production in shoot cultures was 10.15 mg g−1 DW, 6.08 mg g−1 DW and 5.78 mg g−1 DW, respectively, indicating a 1.75-fold increase in bacoside accumulation in airlift bioreactors (Table 4). In another series of tests, Sharma et al. (2019b) compared the outcomes of bottle bioreactors (1 l capacity) and balloon-type bubble bioreactors (2 l capacity) with shake flask cultures (1 l capacity) receiving MS media with 1 mg l−1 BAP. Liquid cultures outperformed solid cultures in terms of biomass accumulation and bacoside production, according to their findings. In agitated flask, bottle bioreactor and balloon-type bubble bioreactors, the quantity of bacoside (bacoside A3 + bacoside A2) accumulated was 115.76 mg l−1 (5.62 mg g−1 DW), 180.18 mg l−1 (5.62 mg g−1 DW) and 321.95 mg l−1 (9.34 mg g−1 DW), respectively. The above findings imply that BM shoots can be grown in bioreactors, allowing for increased shoot biomass accumulation and bacoside synthesis.
Liquid cultures in bioreactors are a good way to propagate plants on a large scale, produce shoot and root biomass and even produce important bioactive substances. Liquid cultures have several advantages, including an easy production system and a quick scale-up process; easy handling, which reduces labour costs; close contact of explants/growing tissues with medium, allowing quick uptake of nutrients for rapid growth; and forced aeration in liquid cultures which favours culture growth by providing a gaseous environment to the growing explants (Murthy et al. 2021). Shoot cultures have been advocated as continuous immersion cultures, transient immersion cultures and spraying methods (Werner et al. 2018). For hairy root cultures, nutrient mist, temporary immersion, wave mixing, bubble column, airlift and disposable bioreactors are recommended (Werner et al. 2018). Although mixing, aeration and shear stress are important elements in bioreactors, the kind of cultures, such as cell cultures, shoot cultures, hairy and adventitious root cultures, influences the choice of bioreactor system. As a result, for the cultivation of BM shoots airlift bioreactors or ebb and flood bioreactors might be useful, and in these bioreactors, several bioprocess parameters such as aeration, shear stress and gaseous parameters should be investigated.
Based on the above studies, it is possible to do a comparison of various factors affecting the accumulation of bacoside A in the cell, shoot and hairy root cultures of BM. MS medium was found to be good for the accumulation of bacoside A (14.04 mg g−1 DW; Seth et al. 2020) when compared to Gamborg’s medium (6.58 mg g−1 DW; Koul and Mallubhotla 2020; Table 1). The salt strength of Gamborg’s medium, especially nitrogen concentration, is considerably lower; thus, the medium with higher salt strength is optimal for cell suspension cultures of BM. Of the various growth regulators tested by various researchers, benzyl amino purine (BAP)/KIN alone or in combination with NAA was found to be appropriate for cell/shoot/hairy root suspension cultures (Tables 1, 2, 3, and 4). Exogenous supply of growth regulators is necessary for the growth of the cells or organ and accumulation of metabolites (Murthy et al. 2021). It was Seth et al. (2020) who optimized cell suspension cultures of BM using response surface methodology and MS medium supplemented with BAP (1.2 mg l−1) in combination with NAA (0.2 mg l−1), and maintenance of cultures for the 18-h/6-h light and dark photoperiod was found optimal for bacoside production (14.04 mg g−1 DW). Comparison of macro-elements, especially nitrogen (NH4+/NO3−) concentration, was tested on the accumulation of bacoside A in shoot cultures of BM, and elevated levels of nitrogen (14.48 mM/37.60 mM of NH4+/NO3−) were suitable for accumulation of bacoside A (27.11 mg g−1 DW; Naik et al. 2011; Table 2). Ahire et al. (2014) studied the effect of potassium chloride (KCl) and CaCl2–induced stress on in vitro shoot cultures of BM (0 mM, 50 mM, 100 mM, 200 mM) and reported accumulation of free proline, glycine, betaine and total sugars in BM shoots with increased concentrations of KCl and CaCl2. They also reported accumulation of higher antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and guaiacol peroxidase (GPX) in shoot cultures with elevated levels of KCl and CaCl2. Ahire et al. (2014) reported the positive effects on bacoside A accumulation with an elevated accumulation of osmolytes and antioxidant enzymes. Comparative analysis of cell, shoot and hairy root cultures on the accumulation of bacoside A suggests that shoot cultures are superior to cell and hairy root suspension cultures (Tables 1, 2 and 3). Optimized cell and shoot cultures showed accumulation of 14.04 mg g−1 and 35.3 mg g−1 DW bacoside A, respectively (Seth et al. 2020; Parale et al. 2010); in contrast, hairy roots accumulated 10.2 to 16.14 mg g−1 DW bacoside A with optimized culture conditions (Bansal et al. 2015). Of the various growth additives tested for accumulation of bacoside A, supplementation of anthranilic acid (0.1 mg g−1) and 100 µM pyruvic acid to the shoot cultures favoured the accumulation of 28.9 mg g−1 and 35.3 mg g−1 DW of bacoside A, respectively (Muszynska et al. 2016; Parale et al. 2010). Largia et al. (2016) experimented with the effect of elicitors such as methyl jasmonate (MJ) and SA individually and in combination (25 µM, 50 µM, 75 µM, 100 µM and 150 µM) for 3 weeks. They have reported increased bacoside A accumulation (269.71 mg g−1 DW) with the treatment of BM shoot cultures with a combination of 25 µM MJ and 25 µM SA treated for a 3-week duration. Further, the above studies have demonstrated that the supplementation of elicitors or salt stress has triggered signaling pathways and probably plays an important role in triggering bacoside biosynthetic genes and facilitating increased accumulation of bacoside A (Fig. 4). Recently, methyl jasmonate–induced expression levels of cinnamoyl CoA dehydrogenase, caffeoyl-CoA methyltransferase, HMG-CA reductase, squalene monooxygenase, isoflavone 2-hydroxylase and geraniol 10-hydroxylase-1 genes have been demonstrated in BM (Jeena et al. 2017, 2021).
Gupta et al. (2017) demonstrated a beneficial effect of chitinolytic microbes, viz. Chitiniphilus sp. MTN22 and Streptomyces sp. MTN14, singly as well as in combination at on the field-cultivated BM plants. They established up-regulation of gene expression of the bacoside biosynthetic pathway (3-hydroxy-3-methylglutaryl coenzyme A reductase, mevalonate diphosphate decarboxylase and squalene synthase) with the augmentation of these microbes as a defense mechanism, which is accountable for a 1.5-fold increment in bacoside A production. These results validate the use of beneficial microbes. Chitiniphilus sp. MTN22 and Streptomyces sp. MTN14 could be used for co-cultivation with in vitro cultures of BM which will boost the accumulation of bacoside A. Comparative analysis of field cultivation of BM and in vitro cultures, for the production bacosides, depicts that in vitro cultivation of BM shoots has advantages because it is responsible for continuous production of BM biomass and consistent production of bacosides. Field cultivation may be affected by edaphic and seasonal factors which account for variations in bacoside accumulation of field-grown plants.
Bacoside biosynthetic pathway and metabolic engineering
The mediators in terpenoid biosynthesis in plants are the products of the methylerythritol phosphate (MEP) and mevalonic acid (MVA) pathways, such as 3,3-dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) (Fig. 3). At the beginning of the reaction, acetyl-CoA is transformed to mevalonic acid in many phases. Mevalonic acid is phosphorylated further to 5-phosphomevalonate, which is then transported to the peroxisome and transformed to an isoprene unit (IPP). The MEP pathway, on the other hand, occurs in plastids and leads to the manufacture of IPP skeleton from 1-deoxy-d-xylulose 5-phosphate (DOXP) via the production of 2-C-methyl-d-erythritol 4-phosphate. Geranyl pyrophosphate (GPP) synthase catalyzes the condensation of DMAPP with one IPP molecule to produce GPP. Alternatively, geranylgeranyl pyrophosphate (GGPP) synthase involves one DMAPP in condensation with IPP to produce GGPP. These prenylated pyrophosphates are further changed by particular enzymes for the formation of monoterpenes and diterpene carbon skeletons (Lu et al. 2020). Head-to-tail condensation of two IPP moieties with DMAPP results in the formation of farnesyl pyrophosphate (FPP), a sesquiterpene precursor. Squalene synthase catalyzes head-to-head condensations of two FPP units to produce the triterpene precursor squalene. Specific oxidosqualene cyclases (OSCs) epoxidize squalene to 2,3-oxidosqualene, which is then converted to tetracyclic or pentacyclic structures to produce the different triterpenes. Later, cytochrome P450 (CYP450)-dependent monooxygenases and glycosyltransferases (GTs) are involved in the oxidation, hydroxylation and glycosylation of triterpenoid saponins and sterols to produce triterpenoid saponins and sterols (Lu et al. 2020).
The isolation and characterization of genes from BM were done by several groups of scientists. Vishwakarma et al. (2012, 2013a, 2013b, 2015) and Kumari et al. (2014) effectively identified the genes for acetyl-CoA C-acetyltransferase (BmAACT), mevalonate kinase (BmMK), mevalonate 5-pyrophosphate decarboxylase (BmMDD) and farnesyl diphosphate synthase (BmFPS) (Kumari et al. 2015). In addition, key genes such as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, FPP synthase and squalene synthase (SQS) were cloned to plasmid (pCMBIA 1301 binary vector) and then introduced to BM plants using Agrobacterium-mediated transformation (Kumari et al. 2015). BmHMGR, BmFPS and BmSQS genes were overexpressed in transformed plants. They also discovered that BmFPS-altered lines accumulate more bacosaponin C (2.7–6.2-fold) and bacoside A (3.7–5.9-fold). These findings show that developing an elite line of BM-overexpressing pathway genes can help researchers better understand the mechanism of bacoside biosynthesis and that these lines can also be used to increase bacoside output using cell and organ culture methods. Jeena et al. (2017, 2021) analysed the transcriptome of BM and found possible genes involved in the manufacture of triterpenoid saponins. They used quantitative reverse transcription polymerase chain reaction (qRT-PCR) to show tissue-specific differential expression of terpenoid biosynthesis genes. They also found transcripts that were specifically involved in the production of triterpenoid sapogenins. The knowledge gained from transcriptome analysis will be used to clone genes into BM via metabolic engineering.
Conclusions and future perspectives
BM is a superb medicinal plant that has been used in Ayurveda for 3000 years to relieve anxiety and improve intelligence and memory. Dammarane triterpenoid glycosides or bacosides extracted from BM have shown pharmacological activity and could be used to treat Parkinson’s disease, Alzheimer’s disease, epilepsy and schizophrenia. Bacoside content varies greatly among natural populations, and demand for this plant has risen steadily over time. As a result, different researchers have attempted to generate bacoside in in vitro cell and organ cultures. Cell, tissue and organ culture techniques have advanced significantly, and researchers have even attempted to generate BM shoots in bioreactors. Despite improvements, some elements of cell/organ culture procedures have been forgotten, such as selecting a superior genotype/plant and explant source, cell/organ line selection and optimization of culture medium, growth regulators and nutrition elements. Supplementing cultures with precursors that are intermediary compounds as well as particular elicitation of cultures employing singling molecules (such as methyl jasmonates and salicylic acid treatments) are all aspects that should be fully investigated. For the development of cells, shoots and roots, research is needed on the selection of bioreactors and the optimization of bioprocess parameters. Furthermore, work on the genomics and proteomics of BM is critical; such research will aid in the up-regulation of metabolic pathways, the redirection of common precursors and the silencing/down-regulation of pathways. The quality, safety and efficacy profiles of raw material derived from in vitro growth are critical (Murthy et al. 2018).
References
Ahire ML, Laxmi S, Walunj PR, Kavi Kishor PB, Nikam TD (2014) Effect of potassium chloride and calcium chloride induces stress on in vitro cultures of Bacopa monnieri (L.) Pennell and accumulation of medicinally important bacoside A. J Plant Biochem Biotechnol 23:366–378
Banerjee S, Anand U, Ghosh S, Ray D, Ray P, Nandy S, Deshmukh GD, Tripathi V, Dey A (2021) Bacosides from Bacopa monnieri extract: an overview of the effect on neurological disorders. Phytotherapy Res 35:5668–5679
Bansal M, Reddy MS, Kumar A (2017) Optimization of cell growth and bacoside-A production in suspension cultures of Bacopa monnieri (L.) Wettst. using response surface methodology. In Vitro Cell Dev Biol Plant 53:527–537
Bansal M, Kumar A, Reddy MS (2015) Production of bacoside A, a memory enhancer from hairy root cultures of Bacopa monnieri (L.) Wettst. J Appl Res Med Aromat Plants 2:92–101
Bansal M, Kumar A, Reddy MS (2014) Influence of Agrobacterium rhizogenes strains on hairy root induction and ‘bacoside-A’ production from Bacopa monnieri (L.) Wettst. Acta Physiol Plant 36:2793–2801
Bhandari P, Sendri N, Devidas SB (2020) Dammarane triterpenoid glycosides in Bacopa monnieri: a review on chemical diversity and bioactivity. Phytochemistry 172:112276
Chatterji N, Rastogi RP, Dhar ML (1965) Chemical examination of Bacopa monniera Wettst. Part II: the constitution of bacoside A. Indian J Chem 3:24–29
Deepak M, Sangli GK, Arun PC, Amit A (2005) Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem Anal 16:24–29
Devendra SPS, Preeti B, Santanu B, Gajanan D, Rupesh D (2018) Brahmi (Bacopa monnieri) as functional food ingredient in food processing industry. J Pharmacogn Phytochem 7:189–194
Dey D, Hazra AK, Nongdam P, Nandy S, Tikendra L, Mukherjee A, Banerjee S, Mukherjee S, Pandey DK (2019) Enhanced bacoside content in polyamine treated in vitro raised Bacopa monnieri (L.) Wettst. S Afr J Bot 123:259–269
Espinosa-Leal CA, Puente-Garza CA, Gracia-Lara S (2018) In vitro plant tissue culture: means for production of bioactive compounds. Planta 248:1–18
Gupta R, Singh A, Srivastava M, Singh V, Gupta MM, Pandey R (2017) Microbial modulation of bacoside A biosynthetic pathway and systemic defense mechanism in Bacopa monnieri under Meloidogyne incognita stress. Sci Rep 7:41867
Gutierrez-Valdes N, Hakkinen T, Lemasson C, Guillet M, Oksman-Caldentey KM, Ritala A, Cardon F (2020) Hairy root cultures – a versatile tool with multiple applications. Front Plant Sci 11:33
Jauhari N, Bhardwaj R, Sharma N, Bhardvaja N (2019) Assessment of bacoside production, total phenol content and antioxidant potential of elicited and non-elicited shoot cultures of Bacopa monnieri. J Environ Sustain 2:441–453
Jeena GS, Kumar S, Shukla RK (2021) Characterization of MYB35 regulated methyl jasmonate and wound responsive Geraniol 10-hydroxylase-1 gene from Bacopa monnieri. Planta 253:89
Jeena GS, Fatima S, Tripathi P, Udpadhyay S, Shukla RK (2017) Comparative transcriptome analysis of shoot and root tissue of Bacopa monnieri identifies potential genes related to triterpenoid saponin biosynthesis. BMC Genomics 18:490
Koul A, Mallubhotla S (2020) Elicitation and enhancement of bacoside production using suspension cultures of Bacopa monnieri (L.) Wettst. 3 Biotech 10:256
Kumari U, Vishawakarma RK, Gupta N, Ruby SMV, Khan BM (2015) Efficient shoots regeneration and genetic transformation of Bacopa monniera. Physiol Mol Biol Plants 21:261–267
Kumari U, Vishwakarma RK, Sonawane P, Abbassi S, Khan BM (2014) Biochemical characterization of recombinant mevalonate kinase from Bacopa monnieri. Int J Biol Macromol 72:776–783
Largia MJV, Satish L, Johnsi R, Shipha J, Ramesh M (2016) Analysis of propagation of Bacopa monnieri (L.) from hairy roots, elicitation and bacoside A contents of Ri transformed plants. World J Microbiol Biotechnol 32:131
Leonard J, Seth B, Sahu BB, Singh VR, Patra N (2018) Statistical optimization for enhancing bacoside-A production in plant cell cultures of Bacopa monnieri. Plant Cell Tiss Organ Cult 133:203–214
Lu Y, Jun L, Juan W, Wen-Yuan G (2020) Advances in biosynthesis of triterpenoid saponins in medicinal plants. Chinese J Nat Med 18:417–424
Majumdar S, Garai S, Jha S (2012) Use of the cryptogein gene to stimulate the accumulation of Bacopa saponins in transgenic Bacopa monnieri plants. Plant Cell Rep 31:1899–1909
Majumdar S, Garai S, Jaha S (2011) Genetic transformation of Bacopa monnieri by wild-type strains of Agrobacterium rhizogenes stimulates the production of Bacopa saponins in transformed calli and plants. Plant Cell Rep 30:941–954
Micheli L, Spitoni S, Mannelli LDC, Bilia AR, Ghelardini C, Pallanti S (2020) Bacopa monnieri as augmentation therapy in the treatment of anhedonia, preclinical and clinical evaluation. Phytother Res 34:2331–2340
Muszynska B, Lojewski M, Sulkowska-Ziaja K, Szewczyk A, Gdula-Argasiriska J, Halaszuk P (2016) In vitro cultures of Bacopa monnieri and an analysis of selected groups of biologically active metabolites in their biomass. Pharm Biol 54:2443–2453
Murthy HN, Dalawai D, Bhat MA, Dandin VA, Paek KY, Park SY (2021) Biotechnological production of useful phytochemicals form adventitious root cultures. In: Ramawat KG, Ekiert HM, Goyal S (eds) Plant cell and tissue differentiation and secondary metabolites: fundamentals and applications. Springer, Cham, pp 469–485
Murthy HN, Dandin VS, Park SY, Paek KY (2018) Quality, safety and efficacy profiling of ginseng adventitious root produced in vitro. Appl Microbiol Biotechnol 102:7309–7317
Naik PM, Manohar SH, Praveen N, Upadhya V, Murthy HN (2012) Evaluation of bacoside A content in different accessions and various organs of Bacopa monnieri (L.) Wettst. J Herbs Spices Med Plants 18:387–395
Naik PM, Manohar SH, Murthy HN (2011) Effects of macro elements and nitrogen source on biomass accumulation and bacoside A production from adventitious shoot cultures of Bacopa monnieri (L.). Acta Physiol Plant 33:1553–1557
National Medicinal Plant Board (NMPB) (2021) Department of Science and Technology, Government of India http://www.nmpb.nic.in/prioritisedmedicinalplants.htm
Parale A, Barmukh R, Nikam T (2010) Influence of organic supplements on production of shoot and callus biomass and accumulation of bacoside in Bacopa monniera (L.) Pennell. Physiol Mol Biol Plants 16:167–175
Paul P, Sarkar S, Jha S (2015) Effects associated with insertion of cryptogenin gene utilizing Ri and Ti plasmids on morphology and secondary metabolites are stable in Bacopa monnieri-transformed plants grown in vitro and ex vitro. Plant Biotechnol Rep 9:231–245
Praveen N, Naik PM, Manohar SH, Nayeem A, Murthy HN (2009) In vitro regeneration of brahmi shoots using semisolid and liquid cultures and quantitative analysis of bacoside A. Acta Physiol Plant 31:733–728
Rahman LU, Verm PC, Singh D, Gupta MM, Banerjee S (2002) Bacoside production by suspension cultures of Bacopa monnieri Pennell. Biotechnol Lett 24:1427–1429
Seth B, Shaoo KK, Aravind KR, Shau BB, Sing VR, Patra N (2020) Statistical optimization of bacoside A biosynthesis in plant cell suspension cultures using response surface methodology. 3 Biotech 10:264
Shalini VT, Neelakanta SJ, Sriranjini JS (2021) Neuroprotection with Bacopa monnieri-a review of experimental evidence. Mol Biol Rep 48:2653–2668
Sharma R, Matins N, Kuca K, Chaudhary A, Kabra A, Rao MM, Prajapati PK (2019a) Chyawanprash: a traditional Indian bioactive health supplement. Biomolecules 9:161
Sharma M, Koul A, Ahuja A, Mallubhotia S (2019b) Suitability of bench scale bioreactor system for shoot biomass production and bacoside biosynthesis from Bacopa monnieri (L.). Eng Life Sci 2019:1–7
Sharma M, Gupta R, Khajuria RK, Mallubhotia S, Ahuja A (2015) Bacoside biosynthesis during in vitro shoot multiplication in Bacopa monnieri (L.) Wettst. grown in Growtek and airlift bioreactors. Indian J Biotechnol 14:547–551
Shrivastava N, Rajani M (1999) Multiple shoot regeneration and tissue culture studies on Bacopa monnieri (L.) Pennell. Plant Cell Rep 18:919–923
Vishwakarma RK, Patel K, Sonawane P, Kumari U, Singh S, Ruby AS, Agarwal DC, Tsay HS, Khan BM (2015) Squalene synthase gene from medicinal herb Bacopa monniera: molecular characterization, differential expression, comparative modeling, and docking studies. Plant Mol Biol Report 6:1675–1685
Vishwakarma RK, Sigh S, Ruby SP, Shrivastava S, Kumari U, Santhosh Kumar RJ, Khan BM (2013a) Molecular cloning, biochemical characterization, and differential expression of an acetyl-CoA C-acetyltransferase gene (AACT) of Brahmi (Bacopa monniera). Plant Mol Biol Report 31:547–557
Vishwakarma RK, Sonawane P, Singh S, Kumari U, Ruby KBM (2013b) Molecular characterization and differential expression studies of an oxidosqualene cyclase (OSC) gene of Brahmi (Bacopa monniera). Physiol Mol Biol Plant 19:547–553
Vishwakarma RK, Patel KA, Soawane P, Singh S, Ruby KU, Agarwal DC, Khan BM (2012) Molecular characterization of farnesyl pyrophosphate synthase from Bacopa monniera by comparative modeling and docking studies. Bioinformation 8:1075–1081
Werner S, Maschke RW, Eibl D, Eibl R (2018) Bioreactor technology for sustainable production of plant cell-derived products. In: Pavlov A, Bley T (eds) Bioprocessing of plant in vitro systems. Reference series in phytochemistry, Springer, Cham. pp. 413-432
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murthy, H.N. Biotechnological production of bacosides from cell and organ cultures of Bacopa monnieri. Appl Microbiol Biotechnol 106, 1799–1811 (2022). https://doi.org/10.1007/s00253-022-11834-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11834-0