Abstract
Microorganisms are indispensable in the food industry, but wild-type strains hardly meet the current industrial demands due to several undesirable traits. Therefore, microbial strain improvement offers a critical solution to enhance the food industry. Traditional techniques for food microbial improvement, such as the use of chemical mutagens and manual isolation/purification, are inefficient, time-consuming, and laborious, restricting further progress in the area of food fermentation. In this review, the applications of novel mutagenesis and screening technologies used for the improvement of food microbes were summarized, including random mutagenesis based on physical irradiation, microbial screening facilitated by a microtiter plate, fluorescence-activated cell or droplet sorting, and microscaled fermentation in a microtiter plate or microbioreactor. In comparison with conventional methods, these new tools have the potential in accelerating microbial strain improvement and their combined applications could create a new trend for strain development. However, several problems that could affect its potential application may include the following: the lack of specific mutagenesis devices and biosensing systems, the insufficient improvement of the mixed culture system, the low efficiency when using filamentous fungi and flocculating bacteria, and the insufficient safety assessment on harnessing genome-editing technology. Therefore, future works on strain improvement remain challenging for the food industry.
Similar content being viewed by others
Introduction
Microorganisms are indispensable in the food industry since they are suppliers of food and drinks, additives, and preservatives. They also serve as “chefs” conferring attractive food flavors (Kum et al. 2015) and aroma (Ardo 2006) that are essential in the culinary industry. In addition, the use of probiotics as a food supplement demonstrates notable health improvement by boosting the growth of human gastrointestinal microflora (Pandey et al. 2015). Altogether, these contributions imply that the food industry heavily relies on microorganisms and microbial biotechnology.
Wild-type (WT) microbial strains can hardly meet industrial demands because of their undesired traits such as low yield, low tolerance, low stability, or abundant by-products in some cases. Researchers have developed various techniques, such as genetic engineering, cellular fusion/hybridization, or adaptive evolution to do strain improvement. However, the applications of genetic modification tools including transposon mutagenesis, staggered extension, mining of novel genes, and random chimeragenesis are restricted in food microorganisms and in the food industry because of safety and risk concerns (Félix et al. 2019; Karabín et al. 2018). Food safety on genetically modified organisms (GMOs) remains a controversial issue among policymakers and consumers (Karabín et al. 2018; von Wright and Bruce 2003). What’s more, the genetic, even cellular and physiological information of most food microbial strains is different from that of lab strains. Thus, it is not suitable to employ techniques that are based on genetic manipulation for food microbe breeding. Besides, although cellular fusion/hybridization and adaptive laboratory evolution techniques are efficient (Li et al. 2017; Cao et al. 2012), they are laborious and time-consuming.
Of all the existing techniques designed for strain improvement, the use of random mutagenesis demonstrates notable advantages over the other technologies for the improvement of food microorganisms. First, random mutagenesis is independent of the host cell’s genetic information making it efficient for microbes with unknown genomic backgrounds; thus, it is suitable for most of the food microorganisms. Second, this tool allows the generation of a mutant library with high genetic diversity, and its manipulation is relatively simple and cost-effective. Finally, it is not involved to controversial GMO issue. Altogether, random mutagenesis represents one of the most useful tools for developing and improving food microorganisms.
A critical step involved in mutagenesis is to select and evaluate desired mutants from the mutagenic library. However, conventional mutant screening is still heavily dependent on shake-flask culturing or manual selection in the food industry (Aleem et al. 2018; Spadiut et al. 2010), which is tedious and inefficient. It becomes far more difficult recently with the emergence of novel mutagenesis technologies that are highly efficient. In addition, assessment of selected mutants and optimization of bioprocessing for industrial scale represent a great challenge. Although flask-culturing remains dominating, this system only offers the “end-point” data and can hardly provide reproducible and reliable parameters that are crucial for the scale-up. Moreover, a scale-up system that relies on a large amount of culture media and expensive nutrient requirements would exacerbate the cost of production affecting downstream prices.
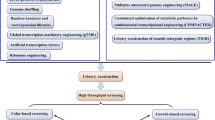
Recently, novel technologies for mutagenesis, screening, and microscale cultivation have accelerated the strain development of industrial microbes (Fig. 1). In contrast, the strain improvement in the food industry still relies heavily on conventional techniques, holding back its potential development. In this review, the recent advances on new mutagenesis and techniques for food microbial screening and cultivation are summarized, and further prospects and trends in strain improvement are discussed. The aim of this review is to address the importance of these novel techniques and to accelerate the food microorganism development.
The application of novel technologies for microbial strain improvement in the food industry. The mutagenic library can be created by fast, safe, and efficient mutagenesis technologies, including ARTP, high-LET HIB, LEIP, HEPEB, etc. The mutants with the desired trait are selected/enriched via sensitive, automatic, and high-throughput screening technologies. Then the enriched mutants are subject to microscale cultivation coupling with online monitoring, acquiring scalable and reliable bioprocessing parameters. These optimized parameters enable the scale-up fermentation
Novel mutagenesis technologies
Chemical mutagens, ultraviolet (UV) irradiation, X-/γ-rays irradiation, and 60Co radiation are the common techniques used to facilitate conventional mutagenesis. Although chemical and UV-mediated mutagenes are prevalent in the food industry, they pose risk to the health of operators (Table 1). Moreover, they are laborious and consume too many media. In this review, only the novel techniques promising in strain improvement in food industry are introduced.
Atmospheric and room temperature plasma-mediated mutagenesis
The demand for mutagenesis techniques that are highly efficient and safe for human health is increasing in the food industry (Table 1). One of them is the atmospheric and room temperature plasma (ARTP)-intermediated mutagenesis, which was developed through collaboration between teams of researchers from Tsinghua University in China (Zhang et al. 2014). Based on the principle of atmospheric pressure radiofrequency glow discharge, the high energy released during the formation of plasma gives rise to DNA mutations (Zhang et al. 2015c). In comparison with conventional methods, ARTP mutagenesis is user-friendly, safe, and fast, thereby generating higher mutation rate under room temperature (RT) conditions that are desirable for microbial growth. Such advantages allow ARTP mutagenesis to be applied widely to improve the traits of numerous microbial species (Li et al. 2015; Tan et al. 2015; Wang et al. 2014; Zhang et al. 2018a).
ARTP mutagenesis exhibits high efficiency in boosting food production as evident in several studies that show its successful application in developing strains of food microorganism (Table 2). Recently, ARTP mutagenesis has demonstrated its power in the overproduction of organic acid and/or fatty acid. In ARTP-mutated Mortierella alpina, intracellular arachidonic acid (ARA) can increase by nearly 2-folds and accounts for a relative increase by 6.65% from the total fatty acids (Li et al. 2015). In ARTP-treated Yarrowia lipolytica strains, the titers of α-ketoglutaric acid showed an increase by 51.8 and 45.4% in a 500-mL flask and 3-L reactor, respectively, when compared to the titers produced by the parental WT strain (Zeng et al. 2015). In Bacillus coagulans, the production of l-lactic acid by ARTP mutants increases substantially by over 40% in 5-L bioreactors (Lv et al. 2016). Microbes are also important suppliers of docosahexaenoic acid (DHA) whereby its health and clinical benefits trigger an increasing demand in the food and health industries. Recently, an ARTP mutant strain of Schizochytrium sp. can produce 14.0 g/L DHA after optimizing the Fe2+ supplementation in shake-flask culture. Its lipid and DHA contents are 31 and 26% higher than the wild-type Schizochytrium, respectively (Zhao et al. 2018). In acetic acid bacteria (AAB) Acetobacter pasteurianu, ARTP treatment does not only lead to an enhanced ethanol tolerance by reducing membrane permeability but also boost significantly the acetic acid titer by nearly 4-folds (Wu et al. 2015). In addition, ARTP mutagenesis also enables high production of amino acids (Cheng et al. 2015; Wang et al. 2015; Zhang et al. 2018b), vitamins (Cai et al. 2018; Xu and Zhang 2017), terpenoids (Qiang et al. 2014; Zhang et al. 2016), polyols (Liu et al. 2017b), enzymes (Jiang et al. 2017; Zhu et al. 2017), polysaccharide (Song et al. 2018), aroma (Wang et al. 2018), and additives (Lin et al. 2016) intended for the food industry (Table 2).
Aside from the enhanced production ability of ARTP mutant strains, the technique can also create mutants that reduce a considerable amount of detrimental by-products, such as the carcinogenic substance ethyl carbamate (EC) from fermented food and urea from fermented beverages. In the soy sauce fermentation, an ARTP mutant strain of Bacillus amyloliquefaciens does not only exhibit enhanced arginine production and salinity tolerance but also can reduce the level of EC and citrulline (an EC precursor) by 19.3 and 15.6%, respectively (Zhang et al. 2017). The same study also suggests that ARTP shows stronger mutagenic effect compared to UV irradiation. In the brewing industry, ARTP mutagenesis could suppress the undesirable metabolites produced by the wild-type Saccharomyces cerevisiae during fermentation. An industrial ARTP mutant S. cerevisiae reduces urea level by 50.6% in rice wine. Such reduction is likely associated with the upregulation of DUR1, DUR2, and DUR3 genes (Cheng et al. 2017). In addition, ARTP technology also contributes to the reduction of toxic methanol (Liang et al. 2014) or acetaldehyde (Liu et al. 2018) in brewing yeast. As shown in Table 2, the improvements of multiple traits occur frequently by using ARTP mutagenesis, suggesting that this tool is efficient and powerful in mutating food microbes.
Iterative ARTP mutagenesis is also a powerful strategy in improving multiple properties. A recent report on the ethanologenic bacterium Zymomonas mobilis shows that a multiplex mutagenesis strategy could create mutants with enhanced acetic acid and low pH tolerance (Wu et al. 2019). It is also demonstrated as a feasible way in food microorganisms. For example, the Sporolactobacillus sp. mutant YBS1-5 (Table 2) obtained from two rounds of ARTP treatment shows further improvement as compared to the mutant from the first ARTP treatment (Sun et al. 2015).
High linear energy transfer heavy ion beam-mediated mutagenesis
Heavy ion beams (HIBs) can generate high linear energy transfer (LET) that induces an increased proportion of DNA double-strand break causing large DNA deletions and/or rearrangements (Hu et al. 2017b; Kazama et al. 2007). Compared to low LET irradiation such as UV rays or X-/γ-rays, high LET-HIB-mediated mutagenesis displays a wider mutation spectrum and a higher mutation frequency, thus emerging as an efficient breeding method (Kazama et al. 2008).
While the continuous use of high LET-HIB for breeding is usually employed in other organisms, its application to food microorganisms is very limited with a few reports focusing mainly on the enhancement of production (Table 3). Using the fungus Aspergillus niger, Hu et al. (2016, 2017a, 2014) successively created some mutants that are citric acid overproducers via carbon ion irradiations. In particular, a mutant strain H4002 could boost the citric acid concentration up to 196.0 g/L with a production level of 3.3 g/L/h, which is currently the highest recorded quantity (Table 3). The same group also improve the l(+)-lactic acid production of Lactobacillus thermophiles by the HIB method. The HIB mutant SRZ50 shows enhanced lactic acid production using either glucose or fructose as a sole carbon source (Hu et al. 2018). Subclone of the bacteria obtained by another HIB treatment further shows substantial improvement in lactic acid production (Jiang et al. 2018).
Breeding of edible mushrooms underlies a great challenge, as it needs the right cultivation method for proper growth and fruiting body production. Recently, the edible mushroom Tricholomamatsutake with improved property has been obtained after gron-ion beams treatment with the LET of 310 keV/μm (Murata et al. 2018). The resulting mutant exhibits not only a dramatic change of phenotype but also shows enhanced capability of degrading dye-linked water-insoluble amylase and cellulose substrates. High LET-HIBs also demonstrate high efficiency in enhancing the production of DHA (Cheng et al. 2016), lipid (Wang et al. 2009a), and cellulase activity (Jiang et al. 2016a) in other genera.
Low-energy ion implantation-mediated mutagenesis
Energetic ions are obtainable under vacuum conditions by acceleration and mass selection. Hypothetically, mutagenesis by low-energy ion implantation (LEIP) is due to the combination of energy absorption, mass deposition, and charge transfer of energetic ions in cells (Gu et al. 2008). In comparison with conventional UV and X-/γ-rays and chemical mutagenesis, LEIP-mediated mutagenesis enables higher mutation rate and wider mutation spectrum (Table 1).
LEIP is a common technique used in plant breeding for decades, but its application in breeding food microbe begins just very recently. So far, its reported applications focus mainly on enhancing enzymatic activities and producing organic/fatty acids (Table 4). Proteases mediate the degradation of proteins into amino acids and small peptides, which contribute greatly to the specific flavor of fermented foods. The LEIP-mediated irradiation of the soy sauce producer Aspergillus oryzae results in significant enhancement of acidic and neutral protease activities during koji fermentation. Furthermore, the mutant A. oryzae exhibits substantial enhancement in protease secretion, protease mRNA expression, and mycelial morphology (Zhao et al. 2012). Aside from proteases, LEIP mutagenesis is also applied successfully to enhance the activity of xylanase (Li et al. 2007), thermostable α-amylase (Li et al. 2011), chitosanase (Su et al. 2006), and lipase (Ji et al. 2008). Moreover, LEIP mutant microbe enhances the production of lactic acid (Li et al. 2012b; Wang et al. 2009b), DHA (Fu et al. 2016), 1,3-dihydroxyacetone (Lin et al. 2016), sophorolipids (Li et al. 2012a), astaxanthin (Liu et al. 2008), and glutathione (Qian et al. 2013). These applications demonstrate the strength of LEIP in the breeding of many food microorganisms. The recent development of ion implantation as a novel way to introduce DNA element into the cells displays great potential in microbial breeding in the future (Gu et al. 2008).
Other techniques
The strain improvement for food microbes in the future is also possible with the use of other physical techniques to induce mutagenesis. For instance, a high-energy pulse electron beam (HEPEB)-mediated technology can trigger substantial DNA double-strand breaks (DSBs) with little effect on the cellular membrane integrity and enzymatic activity. Recent works on HEPEB show great potential in food microbiology, particularly in microbial breeding. For instance, this technique enhances the tolerance and ethanol production of S. cerevisiae (Zhang et al. 2012a, b, 2013). Another promising tool in microbial breeding is the application of laser technology. The technology generates heat, electricity, pressure, and magnet that allow energy accumulation of DNA molecules to an active state and subsequently trigger chemical and/or physical changes in the DNA, such as strand breaking or cross-linking (Liu et al. 2013; Yu et al. 2010).
Screening technology
Mutagenesis is just the first step in the microbial breeding process. Advanced mutagenesis technology creates innumerous mutants. However, an obvious gap between efficient mutagenesis and microbial strain screening makes identification of the mutants of interest from a mutated library much more challenging. Conventional screening relies heavily on manual isolation/purification and flask culture, which are laborious, tedious, and inefficient. While the microbial selection is relatively straightforward when choosing the obvious phenotype (e.g., antibiotic/nutrition/tolerance selective pressures), the selection of a nonobvious phenotype becomes more arduous, thus requiring new and efficient screening techniques. In the succeeding texts, we discuss current microbial screening technologies that show great potential in improving the selection of desirable food microorganisms.
Microtiter plate-based screening
To date, microtiter plate (MTP)-based screening technology is favored because of its moderate to high throughput and automation abilities and cost efficiency, making it a suitable technology platform for preliminary screening (Long et al. 2014). A screening indicator is a key factor to allow the high sensitivity of MTP-based screening. General screening techniques usually use a single indicator based on the colorimetric assay (Rühmann et al. 2015). However, it is difficult to meet the demands for both high sensitivity and accuracy. A prevalent screening procedure is usually comprised of preliminary and secondary steps. The pH is a reliable and versatile indicator for the preliminary screening of those microbes that alter the pH or generate organic acids. Zeng et al. (2015) developed an HTS method based on pH change to select Y. lipolytica mutants with a high yield of α-ketoglutaric acid (α-KG). In the study, ARTP mutant strains undergo two rounds of separate screening using bromocresol and quinaldine red in MTPs. Another team develops a three-step HTS method that combines colony isolation, pH-sensing assay at A616, and l-lactate oxidase (LOD) assay at A500. They used a U-shaped deep-well MTP to screen the B. coagulans mutants overproducing lactic acid (Lv et al. 2016). Consequently, the technique allows the selection of 35 mutants with the desired phenotype from 750 mutant colonies and finally selects two mutants with the highest l-lactic acid yield. In addition, a 24-well U-bottom MTP with bromocresol purple as pH indicator enables the selection of l-lactic acid overproducers from a HIB mutant pool of L. thermophilus (Jiang et al. 2018). On the other hand, Shi et al. (2015) showed an efficient selection of A. niger mutant that overproduces gluconate using a 48-deep-well MTP. The researchers firstly screened mutants using a bromocresol green (a pH indicator), followed by a second screening using gluconate-chelated CuSO4. This method selects three gluconate overproducers out of 1000 mutants. Based on the immobilization of pH-sensing carboxy fluorescein and the pH-insensitive reference sulfohodamin, an HTS method in MTP enables screening of microbes from milk and yogurt where in situ screening is usually difficult (John et al. 2003a). Moreover, pH indicators are used for the preliminary screening of mutants with enhanced enzyme production (Zhu et al. 2017) and reduced by-products (Zhang et al. 2017). The secondary screening commonly uses a colorimetric assay in selecting the specific characteristics of the product of interest.
Fluorescence-activated cell sorting
Flow cytometry (FC) enables counting, monitoring, enriching, and sorting of microbial cells suspended in fluid streams. FC technology uses fluorescence and microfluidic techniques for high-throughput characterization, identification, and screening of food microorganisms. Among them, fluorescence coupled with FC allows fast and real-time screening of the desired microbial strains. Fluorescent signals that correlate with cellular chemical or physical characteristics of the target strain enable microbial selection through fluorescence-activated cell sorting (FACS). For instance, FACS is applied in the screening of strains with a phenotype that overproduced the essential antioxidant glutathione (γ-glutamyl-L-cysteinylglycine, GSH). Selecting a mutant with a high content of GSH is critical. To date, yeast is the main GSH producer in the food industry. S. cerevisiae mutant G-143, a product of ethyl methanesulfonate (EMS) mutagenesis that produces high GSH content, is isolated based on the fluorescence intensity formed by intracellular GSH using monochlorobimane (mBCl) (Wang et al. 2010).
Flow cytometry-based technique using two nucleic acid stains, i.e., SYBR Green II RNA gel and propidium iodide, enables an accurate assessment and cell sorting of Lactobacillus sakei under heat and acid stress conditions (Bonomo et al. 2013). Through this protocol, fluorescent intensities and types could identify cells with different viabilities and conditions. In addition, a combination of FACS and fluorescence in situ hybridization (FISH) using a specific 16S rRNA probe labeled with fluorescein isothiocyanate (FITC) allows the identification and selection of functional acetic acid bacteria in vinegar. This procedure enables relatively short screen time to select strains that have high resistance to acetic acid and/or with a high yield of acetic acid from Komagataeibacter, Acetobacter, and Gluconobacter genera (Trček et al. 2016).
Aside from the heterogeneous probes, native molecular elements are also applicable for FACS. Vitamin B12 (VB12) is widely used in the food industry with increasing demand in the global market. To date, mutagenesis is still a major means to enhance its production, so the selection for high-yield mutants becomes critical. Cai et al. (2018) developed an HTS system for isolating Sinorhizobium meliloti mutants with high VB12 content. In this system, the riboswitch RNA element of btuB, a key gene for VB12 biosynthesis, regulates the expression of GFP and lacI reporter system. This allows the identification and selection of cells based on the positive correlation between intracellular VB12 level and fluorescence intensity. Using this system, the same team isolates an ARTP mutant MC5-2 that is capable of producing VB12 by 21.9% higher than the wild-type strain.
Fluorescence-activated droplet sorting
Droplet microfluidics emerges as a screening technology with high-throughput and high-resolution characteristics. Picoliter-sized aqueous droplets are dispersed in a continuous fluorinated oil phase, allowing stable compartmentalization by surfactants. Theoretically, every variation can be detected and sorted based on its size and associated fluorescence.
Screening microbes producing enzymes represents a challenge, due to the lack of obvious phenotype and low efficiency of the conventional screening. Furthermore, current physiological and biochemical methods used in enzymatic assays are often laborious and expensive. Droplet microfluidics-based screening provides a solution as this tool enables fast screening rate (i.e., thousands of droplets per second) just by using a tiny reaction volume. Sjostrom et al. (2014) developed a fluorescence-activated droplet sorting (FADS)-based HTS to select yeast cells with improved secretion of α-amylase from a whole-genome mutated cell library. The method enables a saturated screening of the mutant library with a great reduction of reagent consumption. In a following study, researchers from the same group selected dozens of S. cerevisiae mutants with enhanced α-amylase secretion ability from UV mutagenesis libraries using two rounds of HTS based on FADS (Huang et al. 2015), demonstrating the reliability of their method. Whole-genome sequencing then revealed 330 mutation sites within 146 protein-encoding genes in the genome that are related to secretion modulation. Finding these unknown but critical loci and genes would have been impossible without the use of FADS.
For filamentous fungi such as A. niger, MTP-based screening is usually neither efficient nor cost-effective. Even FACS-based screening is also problematic because of the oversized A. niger mycelia. By contrast, FADS demonstrates promising results as an efficient technology for the HTS of filamentous fungi. Using the same fluorescein as the two studies mentioned above, a UV-mutated A. niger library with enhanced α-amylase activity is enriched by approximately 200-folds through FADS. In comparison with a UV-mutated reference strain, 37% of the sorted mutants show higher amylase activity (Beneyton et al. 2016).
In addition, FADS-based HTS also demonstrates a powerful capacity to screen lipase overproducers (Qiao et al. 2017), p-coumaric acid-overproducing S. cerevisiae strain (Siedler et al. 2017), or vitamin overproducer lactic acid bacteria (LAB) strain (Chen et al. 2017). A comprehensive review of its application for strain improvement of LAB has been published elsewhere (Chen et al. 2018). Although these examples were not specifically associated with mutagenesis, they demonstrate that droplet microfluidics-based screening is promising for strain improvement in the food industry.
Microscaled cultivation
Although mutant enrichment can be performed through HTS, the selection of the desired mutant microbe for fermentation remains a challenge. On one hand, a lack of kinetics information in flask culture system would likely result in poor reproducibility. On the other hand, it is impossible to assess every mutant of interest in scaled bioreactors. Thus, microscaled cultivation is currently developed and applied in strain improvement for bioprocess monitoring and for control and optimization. Considering fermentation aims to producing at an industrial scale, desirable microscale fermentation needs to meet several basic criteria, such as scalability, reliability, and real-time monitoring. The principles and applications of microscale cultivation in pharmaceutical or industrial microorganisms have been reviewed (Long et al. 2014; Schäpper et al. 2009); hence, this review focuses only on their potential application and development in food microbes.
Online process monitoring
Process monitoring is crucial for fermentation. However, conventional monitoring is based on “off-line” techniques which require sampling out of a reactor to do measurement. In addition, pH and DO values are conventionally measured using electrodes that need calibration. However, modern fermentation processing concerns more about online and noninvasive monitoring with medium to high throughput than ever before. Since MTP-based cultivation can meet the demands of throughput, it is prevalent to develop novel monitoring techniques.
Mutant microbes are desirable materials to investigate intracellular metabolic flux. However, the flux tends to be susceptible by microbial growth status. The combination of microscale cultivation with molecular techniques allows the task to be easier. In lysine-producing mutants of Corynebacterium glutamicum, the MTP-based online monitoring associated with cultivation feeding 13C isotope reveals that changes on kinetics significantly disturbed the metabolic flux for lysine production (Wittmann et al. 2004). Oxygen transfer rate (OTR) is a critical parameter for aerobic fermentation, but it greatly differs between regular MTPs and shaking flasks, and thus, this parameter is a major bottleneck for the scale-down or scale-up of microbial fermentation. A microscale system developed for the amino acid producer Corynebacterium glutamicum shows that biomass and specific growth rate parameters are comparable between MTPs and flask. The system involves a setup for both oxygen-sensitive and reference fluorophores that are immobilized at the bottom of wells and used a 96-well MTP to sense the concentration of dissolved oxygen (DO) (John et al. 2003b).
Recently, a MTP fermentation system called BioLector microbial bioreactor is available in the market. By means of scattered light and fluorescence optic measurement techniques, it can be used to analyze strain phenotype and growth, screen mutants, and/or optimize medium and other fermentation parameters. Compared with conventional bioprocessing tools, BioLector is labor and cost-effective and has high reproducibility. Infrared fluorescent oxygen-sensitive nanoparticle was demonstrated as a reliable method to monitor OTR of Hansenula polymorpha and Gluconobacter oxydans via BioLector-based respiration activity monitoring system (RAMOS) (Jang et al. 2017). The results prove that none of the fluorescence intensities, concentrations, and types disturbed the DO measurement by infrared fluorescence. However, RAMOS is only available in small-scale fermentation such as a flask or milliliter-scale microbioreactors. Flitsch et al. (2016) constructed a 48-well MTP-based μRAMOS device with reduced pneumatic valves and sensors but with a steady well-to-well integration. The cultivation of microbes such as H. polymorpha in this device shows a comparable OTR with the flask culture system. In contrast to a bioreactor, microscale cultivation shows higher efficiency with reduced cost and media consumption.
Process control and optimization
Microscaled cultivation is able to accelerate the optimization of biological and bioprocessing parameters with high throughput. By means of the BioLector microbioreactor platform equipped with the smart design of triggering sampling or dosing by pipetting robot, Rohe et al. (2012) quickly optimized fermentation conditions for high lipolytic activity in C. glutamicum after testing multiple parameters in a mean time such as pH, OTR, inoculation and induction time, specific growth rate, biomass, and enzymatic activity. Aspergillus terreus is a main producer for itaconic acid. Microscaled culture system performed using MTPs simplifies and speeds up the optimization process for itaconic acid production. The cultivation demonstrates the critical roles of CuSO4 and KH2PO4, as well as the dispensability of nitrogen and phosphate for fermentation (Hevekerl et al. 2014).
Scalability and reliability are critical properties for microscale fermentation. In the examples mentioned above, Rohe et al. (2012) found that biomass accumulation and substrate utilization during microscale fermentation were quite similar to that when using a 1-L bioreactor. In 20-L bioreactors, both parameters reduced but still comparable to the smaller-scaled reactors, but the cutinase activities under the optimal conditions were comparable in all scales. Similarly, the results studied by Hevekerl et al. (2014) also demonstrated reliability and scalability of microscale fermentation since the bench-scale fermentation using the optimized medium showed high production of itaconic acid. However, MTP-based cultivation for some filamentous fungi remains problematic. The application of the Duetz-MTP system allows comparison of fungal fatty acid producers Mucor circinelloides, Mo. alpina, and microalga in various scales. Biomass accumulation, lipid content, and ARA production show relative scalability between MTPs, bench-top, and 25-L bioreactor, but the reproducibility varies between techniques (Kosa et al. 2018). In A. niger, 2-phenylethanol (2-PE) production in FlowerPlate MTP cultivation shows a 35% reduction compared with that in flasks, even with the addition of homogenate chemicals (Huth et al. 2017).
Microfermentation
MTP-based system for aerobic fermentation relies on a special well (Funke et al. 2009), a lid (Schleputz and Buchs 2014), and external power to improve oxygen mass transfer. Alternatively, a milliliter-scale bioreactor is equipped with a novel stirrer that enables good mixing and high oxygen transfer rate (Bolic et al. 2016). Magnetic stirring and computational fluid dynamics (CFD) allow one- and bi-directional mixing and estimate the mixing time. This system generates high compatibility and flexibility (0.5–2 mL) in S. cerevisiae and Lactobacillus paracasei. In particular, this is also suitable in the case of filamentous fungi with high-viscosity fermentation. Further findings suggest that viscosity up to 35 mPa did not show any major influence on the oxygen transfer rate and the mixing process. The details on microscale cultivation technology and machines have been reviewed recently (Puskeiler et al. 2005).
In addition, MTP-based fermentation is developed for mixed cultured food. Ethanol and acetic acid levels produced by AAB are critical for the flavor of vinegar. The use of FlowerPlate BioLector allows a high-throughput system for bacterial selection and process investigation in a microscaled setup. Moreover, the design of a custom-made lid coupled to MTP cultivation permits simultaneous online monitoring, automatic sampling and measurement, efficient mass transfer, and prevention from evaporation. The MTP cultivation system could efficiently prevent evaporation of ethanol, acetic acid, and culture media. On top of that, the fermentation performance is comparable to that in a 9-L bioreactor (Schleputz and Buchs 2014).
Anaerobes are a special kind of food producers. So far, the study on miniaturized cultivation system for anaerobic fermentation is rare. A newly developed special MTP-based system, called OVAMO, uses Oxyrase, vacuum, and mineral oil to realize an in situ anaerobic environment in 96-well MTPs (Lam et al. 2018). This cost-effective system enables the growth of probiotics such as Bifidobacterium longum and carbohydrate monitoring in MTPs. The product obtained from OVAMO is comparable with that obtained from anaerobic jars.
Future perspectives
Although modern technologies for strain development are widely applied, some identified challenges could affect its potential application. These challenges may include the lack of specific mutagenesis devices and biosensing systems, the insufficient improvement of the mixed culture system, the low efficiency when using filamentous fungi and flocculating bacteria, and the insufficient safety assessment on harnessing genome-editing technology. Hence, strain improvement in the food industry still needs a lot of work in the future.
Combined mutagenesis
Repetitive treatments by a single mutagenesis technology would likely reduce mutation efficiency. For example, lactic acid production by Sporolactobacillus after the first round of ARTP treatment could boost by nearly 40%, while the second round could add around 11% on its production (Sun et al. 2015). Combined treatment is also efficient to improve the strain of food microbes. Gluconobacter oxydans is an outstanding example where the combined treatment with three mutagenesis techniques generates a significant overproduction of 1,3-dihydroxyacetone. The first process uses UV mutagenesis to select the overproducer strain U-2-115. This strain shows a 48.8% increase of 1,3-dihydroxyacetone production compared to the original one. The second process subjects the U-2-115 mutant into ARTP treatment generating a second mutant A-2-64, which can produce 26.3% more than the first mutant strain. The final process uses LEIP treatment to create the mutant strain I-2-239. This mutant strain shows dihydroxyacetone production of 103.5 mg/mL, i.e., 14.7 and 115.7% increase compared to the strain A-2-64 and the original strain L-6, respectively (Lin et al. 2016).
The combination of mutagenesis technology with other biotechniques shows some promising results as it works synergistically and efficiently. Jin et al. (2018) created an engineered S. cerevisiae strain via ARTP mutagenesis and metabolic engineering. This approach results in high astaxanthin yield of up to 10.1 mg/g dry cell weight (DCW), which is so far the highest yield recorded from a yeast culture using a flask. In B. coagulans, mutants derived from ARTP and ALE treatment can grow in a medium with 80% diluted acid hydrolysates (Jiang et al. 2016b). Moreover, genome shuffling coupled with mutagenesis is also reported to improve the breeding of food microbes (Zhang et al. 2015a).
Development of biosensing systems
Undoubtedly, the biosensing system plays a central role for screening and microscale cultivation for being high throughput, highly sensitive, and reliable and for its ability to monitor microbial culture in real time. The type, development, and potential use of biosensors are reviewed elsewhere (Gredell et al. 2012; Han et al. 2018; Johnson et al. 2017; Mertens and Liese 2004; Schallmey et al. 2014; Shibasaki and Ueda 2014; Zhang et al. 2015b). So far, there are very few progresses on the use of biosensors in the food industry for sensing and/or selecting pH (Casimero et al. 2018), oxygen and cellular viability(Strianese et al. 2009), aroma (Liu et al. 2015), or amino acid producers (Liu et al. 2017a; Mustafi et al. 2012), lagging behind the actual needs of the food industry. So far, real applications of biosensing systems in the industry are rare, but they would be promising in the near future, in particular in pharmaceutical or biotechnological plants producing high value-added products, which have been addressed in recent reviews (Neelam et al. 2019; Lam et al. 2012). However, they have not been widely applicable in the food industry yet. We can predict that microscale fermentation-equipped biosensing systems will be available for screening and process optimization in the food industry, but their cost will be critical to apply in the food industry.
Strain improvement for special food microorganisms
Traditional fermented food by mixed cultures
Mixed microbial cultures play a substantial role in the production of traditional food, including Chinese liquor, pickles, soy sauce, and fermented soya. Because of the great economic, nutritional, and cultural values offered by traditional food, the improvements in their nutritional contents and flavors are increasingly attractive. However, efficient mutagenesis and screening techniques for mixed cultures are much more difficult than for pure microbial cultures, because the mixed cultures form a consortium in which cellular interaction, evolution, and survival are different from pure cultures, and such a complicated consortium rather than a certain individual member confers food diverse flavors, metabolites, and so on. The functions of the microorganisms in mixed cultures are different, so it is hard to unify the mutation and screening directions. Meanwhile, different microorganisms in mixed cultures have different sensitivities to mutation factors, and it is difficult to screen a suitable mutation technique for mixed microbe mutation. A compromised mutagenesis strategy for mixed culture is to mutate a few cultured members and then acclimatize them to a mixed culture condition (Zhang et al. 2015a). However, the strategy does not consider the mixed cultures as an organic whole and neglects the contribution of uncultured or nonisolated microbes in the mixed cultures. In addition, it is infeasible to uncultured but functional species. A desirable mutagenesis technology for mixed cultures should enable in situ and efficient mutation of all microbial members at one time. Therefore, improvement of the mutagenesis techniques must involve the following modifications: treating a larger cell amount, optimizing the treatment time and strength, and maintaining steady mutation efficiency in a solution. On the other hand, HTS will be a primary requirement for mixed cultures though it will be challenging to perform. A recent report on the use of droplet-based microfluidics for the HTS of novel enzyme resources from metagenomic library provides a promising strategy on screening mixed cultures of food microbes (Hosokawa et al. 2015).
Screening and microscale cultivation of filamentous fungi and flocculating bacteria
Filamentous fungi and flocculating bacteria are especially important for fermented food because of their high yield, high tolerance, and easy recovery. However, the formation of biomass agglomerates or flocs makes the screening and assessment more challenging. Oversized pellets and/or uneven phase in the solution constrain the application of screening methods, which are dependent on optical density, surface characteristics (e.g., using FACS), and droplet microfluidics-based technology. Since filamentous fungi are vulnerable to mechanic shearing and tend to generate high viscous culture broth during fermentation, the scalability of microcultivation for fungi still requires improvement. To partly address this concern, a recent study creates a three-dimensional model to stimulate the hyphal growth and OTR of the filamentous fungus Rhizopus oligosporus at a microscale and solid-state fermentation (Coradin et al. 2011). Hence, microscale cultivation significantly guides in understanding any specific fungi even though its robustness remains to be tested.
The genome editing and breeding of food microorganisms
Genome-editing technology using the CRISPR system is a recent powerful tool (Doudna and Charpentier 2014; Hsu et al. 2014). Since it introduces no foreign genetic elements into the host genome, it is regarded as safe and different from genetic modification (GM). So far, most applications of the CRISPR technique were reported in LAB or yeast. Using such technique could efficiently enhance the production of food-grade lactic acid (Jang et al. 2017) or N-acetylglucosamine (Zhou et al. 2019) without introducing antibiotic markers or heterogeneous DNA. During yeast breeding, CRISPR enabled to enhance thermotolerance (Mitsui et al. 2019) or reduce its foam of sake (Ohnuki et al. 2019). In addition, researchers also demonstrated the efficacy and feasibility of applying CRISPR in microbial populations. Barrangou and Notebaart (2019) and Goh and Barrangou (2019) have reviewed advances on using CRISPR techniques to engineer probiotics for the aim of therapy and health, or reshaping microbial populations of the food supply for food safety. In addition, there were reports on developing CRISPR tools in edible mushroom (Binhu and Das 2019) or Lactobacillus plantarum and Lactobacillus brevi (Huang et al. 2019), displaying great potential for strain development in the food industry. However, the study of Leenay et al. (2019) indicated that the precision of CRISPR varied and was likely host-dependent. This result reminds us again to be cautious to the food safety and risk management by the genome-editing techniques (Mays and Nair 2018; Varela and Varela 2019).
References
Aleem B, Rashid MH, Zeb N, Saqib A, Ihsan A, Iqbal M, Ali H (2018) Random mutagenesis of super Koji (Aspergillus oryzae): improvement in production and thermal stability of α-amylases for maltose syrup production. BMC Microbiol 18:200
Ardo Y (2006) Flavour formation by amino acid catabolism. Biotechnol Adv 24(2):238–242
Barrangou R, Notebaart RA (2019) CRISPR-directed microbiome manipulation across the food supply chain. Trends Microbiol 27(6):489–496
Beneyton T, Wijaya IP, Postros P, Najah M, Leblond P, Couvent A, Mayot E, Griffiths AD, Drevelle A (2016) High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci Rep 6:27223
Binhu J, Das A (2019) An edible fungi Pleurotus ostreatus inhibits adipogenesis via suppressing expression of PPAR γ and C/EBP α in 3T3-L1 cells: in vitro validation of gene knock out of RNAs in PPAR γ using CRISPR spcas9. Biomed Pharmacother 116:109030
Bolic A, Larsson H, Hugelier S, Eliasson Lantz A, Krühne U, Gernaey KV (2016) A flexible well-mixed milliliter-scale reactor with high oxygen transfer rate for microbial cultivations. Chem Eng J 303:655–666
Bonomo MG, Milella L, Martelli G, Salzano G (2013) Stress response assessment of Lactobacillus sakei strains selected as potential autochthonous starter cultures by flow cytometry and nucleic acid double-staining analyses. J Appl Microbiol 115(3):786–795
Cai Y, Xia M, Dong H, Qian Y, Zhang T, Zhu B, Wu J, Zhang D (2018) Engineering a vitamin B12 high-throughput screening system by riboswitch sensor in Sinorhizobium meliloti. BMC Biotechnol 18(1):27
Cao X, Song Q, Wang C, Hou L (2012) Genome shuffling of Hansenula anomala to improve flavour formation of soy sauce. World J Microbiol Biotechnol 28:1857–1862
Casimero C, McConville A, Fearon JJ, Lawrence CL, Taylor CM, Smith RB, Davis J (2018) Sensor systems for bacterial reactors: a new flavin-phenol composite film for the in situ voltammetric measurement of pH. Anal Chim Acta 1027:1–8
Chen J, Vestergaard M, Jensen TG, Shen J, Dufva M, Solem C, Jensen PR (2017) Finding the needle in the haystack—the use of microfluidic droplet technology to identify vitamin-secreting lactic acid bacteria. mBio 8:3
Chen J, Vestergaard M, Shen J, Solem C, Dufva M, Jensen PR (2018) Droplet-based microfluidics as a future tool for strain improvement in lactic acid bacteria. FEMS Microbiol Lett 365(23)
Cheng G, Xu J, Xia X, Guo Y, Xu K, Su C, Zhang W (2015) Breeding L-arginine-producing strains by a novel mutagenesis method: atmospheric and room temperature plasma (ARTP). Prep Biochem Biotech 46(5):509–516
Cheng Y, Du G, Zhou J, Chen J (2017) High-throughput screening of mutant strain to reduce the accumulation of ethyl carbamate precursor in rice wine fermentation. Acta Microbiol Sin 57(10):1517–1526
Cheng YR, Sun ZJ, Cui GZ, Song X, Cui Q (2016) A new strategy for strain improvement of Aurantiochytrium sp. based on heavy-ions mutagenesis and synergistic effects of cold stress and inhibitors of enoyl-ACP reductase. Enzyme Microb Tech 93-94:182–190
Coradin JH, Braun A, Viccini G, de Lima Luz LF Jr, Krieger N, Mitchell DA (2011) A three-dimensional discrete lattice-based system for modeling the growth of aerial hyphae of filamentous fungi on solid surfaces: a tool for investigating micro-scale phenomena in solid-state fermentation. Biochem Eng J 54(3):164–171
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1258096
Félix FKC, Letti LAJ, Pereira GVDM, Bonfim PGB, Soccol VT, Soccol CR (2019) L-lysine production improvement: a review of the state of the art and patent landscape focusing on strain development and fermentation technologies. Crit Rev Biotechnol 39(8):1031–1055
Flitsch D, Krabbe S, Ladner T, Beckers M, Schilling J, Mahr S, Conrath U, Schomburg WK, Buchs J (2016) Respiration activity monitoring system for any individual well of a 48-well microtiter plate. J Biol Eng 10:14
Fu J, Chen T, Lu H, Lin Y, Xie X, Tian H, Zheng C, He D (2016) Enhancement of docosahexaenoic acid production by low-energy ion implantation coupled with screening method based on Sudan black B staining in Schizochytrium sp. Bioresour Technol 221:405–411
Funke M, Diederichs S, Kensy F, Muller C, Buchs J (2009) The baffled microtiter plate: increased oxygen transfer and improved online monitoring in small scale fermentations. Biotechnol Bioeng 103(6):1118–1128
Goh YJ, Barrangou R (2019) Harnessing CRISPR-Cas systems for precision engineering of designer probiotic lactobacilli. Curr Opin Biotech 56:163–171
Gredell JA, Frei CS, Cirino PC (2012) Protein and RNA engineering to customize microbial molecular reporting. Biotechnol J 7(4):477–499
Gu S-b, Li S-c, Feng H-y, Wu Y, Yu Z-l (2008) A novel approach to microbial breeding-low-energy ion implantation. Appl Microbiol Biot 78:201
Han L, Zhao Y, Cui S, Liang B (2018) Redesigning of microbial cell surface and its application to whole-cell biocatalysis and biosensors. Appl Biochem Biotech 185(2):396–418
Hevekerl A, Kuenz A, Vorlop KD (2014) Filamentous fungi in microtiter plates—an easy way to optimize itaconic acid production with Aspergillus terreus. Appl Microbiol Biot 98(16):6983–6989
Hosokawa M, Hoshino Y, Nishikawa Y, Hirose T, Yoon DH, Mori T, Sekiguchi T, Shoji S, Takeyama H (2015) Droplet-based microfluidics for high-throughput screening of a metagenomic library for isolation of microbial enzymes. BiosensBioelectron 67:379–385
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157(6):1262–1278
Hu W, Chen J-h, Wang S-y, Liu J, Song Y, Wu Q-f, Li W-j (2016) Changes in the physiological properties and kinetics of citric acid accumulation via carbon ion irradiation mutagenesis of Aspergillus niger. J Zhejiang Univ-SCI B 17(4):262–270
Hu W, Chen J, Wu Q, Li W, Liu J, Lu D, Wang S (2018) The mutagenesis of Lactobacillus Thermophilus for enhanced L-(+)-lactic acid accumulation induced by heavy ion irradiation. Braz Arch Biol Techn 60(0)
Hu W, Li W, Chen H, Liu J, Wang S, Chen J (2017a) Changes in transcript levels of starch hydrolysis genes and raising citric acid production via carbon ion irradiation mutagenesis of Aspergillus niger. PLoS One 12(6):e0180120
Hu W, Li W, Chen J (2017b) Recent advances of microbial breeding via heavy-ion mutagenesis at IMP. Lett Appl Microbiol 65(4):274–280
Hu W, Liu J, Chen JH, Wang SY, Lu D, Wu QH, Li WJ (2014) A mutation of Aspergillus niger for hyper-production of citric acid from corn meal hydrolysate in a bioreactor. J Zhejiang Univ-SCI B 15(11):1006–1010
Huang M, Bai Y, Sjostrom SL, Hallstrom BM, Liu Z, Petranovic D, Uhlen M, Joensson HN, Andersson-Svahn H, Nielsen J (2015) Microfluidic screening and whole-genome sequencing identifies mutations associated with improved protein secretion by yeast. Proc Natl Acad Sci U S A 112(34):E4689–E4696
Huang H, Song X, Yang S (2019) Development of a RecE/T-assisted CRISPR-Cas9 toolbox for Lactobacillus. Biotechnol J 14:1800690
Huth I, Schrader J, Holtmann D (2017) Microtiter plate-based cultivation to investigate the growth of filamentous fungi. Eng Life Sci 17(10):1064–1070
Jang YJ, Seo SO, Kim SA, Li L, Kim TJ, Kim SC, Jin YS, Han NS (2017) Elimination of the cryptic plasmid in Leuconostoc citreum by CRISPR/Cas9 system. JBiotechnol 251:151–155
Ji Y, Tan T, Du L (2008) Study on mutation breeding of the lipase producing strain Rhizopus arrhizus with low energy N+ ion beam implantation. J Biotechnol 136:S337
Jiang A-l, Hu W, Li W-j, Liu L, Tian X-j, Liu J, Wang S-y, Lu D, Chen J-h (2018) Enhanced production of l-lactic acid by Lactobacillus thermophilus SRZ50 mutant generated by high-linear energy transfer heavy ion mutagenesis. Eng Life Sci 18(9):626–634
Jiang B-L, Wang S-Y, Wang Y-C, Chen J-H, Li W-J, Liu J, Hu W, Xiao G-Q, Dong M-Y, Xu F-Q (2016a) A high-throughput screening method for breeding Aspergillus niger with 12C6+ ion beam-improved cellulase. Nucl Sci Tech 28(1):1
Jiang T, Qiao H, Zheng Z, Chu Q, Li X, Yong Q, Ouyang J (2016b) Lactic acid production from pretreated hydrolysates of corn stover by a newly developed Bacillus coagulans strain. PLoS One 11(2):e0149101
Jiang Y, Shang YP, Li H, Zhang C, Pan J, Bai YP, Li CX, Xu JH (2017) Enhancing transglutaminase production of Streptomyces mobaraensis by iterative mutagenesis breeding with atmospheric and room-temperature plasma (ARTP). Bioresour Bioprocess 4(1):37
Jin J, Wang Y, Yao M, Gu X, Li B, Liu H, Ding M, Xiao W, Yuan Y (2018) Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol Biofuels 11:230
John GT, Goelling D, Klimant I, Schneider H, Heinzle E (2003a) pH-sensing 96-well microtitre plates for the characterization of acid production by dairy starter cultures. J Dairy Res 70(3):327–333
John GT, Klimant I, Wittmann C, Heinzle E (2003b) Integrated optical sensing of dissolved oxygen in microtiter plates: a novel tool for microbial cultivation. Biotechnol Bioeng 81(7):829–836
Johnson AO, Gonzalez-Villanueva M, Wong L, Steinbuchel A, Tee KL, Xu P, Wong TS (2017) Design and application of genetically-encoded malonyl-CoA biosensors for metabolic engineering of microbial cell factories. Metab Eng 44:253–264
Karabín M, Jelínek L, Kotrba P, Cejnar R, Dostálek P (2018) Enhancing the performance of brewing yeasts. Biotechnol Adv 36(3):691–706
Kazama Y, Saito H, Fujiwara M, Matsuyama T, Hayashi Y, Ryuto H, Fukunishi N, Abe T (2007) An effective method for detection and analysis of DNA damage induced by heavy-ion beams. Biosci Biotechnol Biochem 71(11):2864–2869
Kazama Y, Saito H, Yamamoto YY, Hayashi Y, Ichida H, Ryuto H, Fukunishi N, Abe T (2008) LET-dependent effects of heavy-ion beam irradiation in Arabidopsis thaliana. Plant Biotechnol 25(1):113–117
Kosa G, Vuoristo KS, Horn SJ, Zimmermann B, Afseth NK, Kohler A, Shapaval V (2018) Assessment of the scalability of a microtiter plate system for screening of oleaginous microorganisms. Appl Microbiol Biot 102(11):4915–4925
Kum SJ, Yang SO, Lee SM, Chang PS, Choi YH, Lee JJ, Hurh BS, Kim YS (2015) Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang). J Agr Food Chem 63(5):1401–1418
Lam KL, Lin S, Liu C, Wu X, Tang S, Kwan HS, Cheung PC (2018) Low-cost method generating in situ anaerobic conditions on a 96-well plate for microbial fermentation in food research. J Agr Food Chem 66(44):11839–11845
Lam E, Male KB, Chong JH, Leung AC, Luong JH (2012) Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol 30(5):283–290
Leenay RT, Vento JM, Shah M, Martino ME, Leulier F, Beisel CL (2019) Genome editing with CRISPR-Cas9 in Lactobacillus plantarum revealed that editing outcomes can vary across strains and between methods. Biotechnol J 14:1700583
Li H, Ma X, Shao L, Shen J, Song X (2012a) Enhancement of sophorolipid production of Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 by low-energy ion beam implantation. Appl Biochem Biotech 167(3):510–523
Li S, Yao J, Yu Z (2007) Studies on mutation breeding of high-yielding xylanase strains by low-energy ion beam implantation. Plasma Sci Technol 9(2):248
Li SC, Zhu ZY, Gu SB, Liu HX, Wang DD (2012b) Screening of a L-lactic acid producing strain of Lactobacillus casei by low-energy ion implantation. Adv Mater Res 455-456:471–476
Li X, Liu R, Li J, Chang M, Liu Y, Jin Q, Wang X (2015) Enhanced arachidonic acid production from Mortierella alpina combining atmospheric and room temperature plasma (ARTP) and diethyl sulfate treatments. Bioresour Technol 177:134–140
Li XY, Zhang JL, Zhu SW (2011) Improved thermostable alpha-amylase activity of Bacillus amyloliquefaciens by low-energy ion implantation. Genet Mol Res 10(3):2181–2189
Li Y, Zeng W, Gou M, Sun Z, Xia Z, Tang Y (2017) Transcriptome changes in adaptive evolution of xylose-fermenting industrial Saccharomyces cerevisiae strains with δ-integration of different xylA genes. Appl Microbiol Biotechnol 101:7741–7753
Liang M-H, Liang Y-J, Chai J-Y, Zhou S-S, Jiang J-G (2014) Reduction of methanol in brewed wine by the use of atmospheric and room-temperature plasma method and the combination optimization of malt with different adjuncts. J Food Sci 79(11):M2308–M2314
Lin X, Liu S, Xie G, Chen J, Li P, Chen J (2016) Enhancement of 1,3-dihydroxyacetone production from Gluconobacter oxydans by combined mutagenesis. J Microbiol Biotechnol 26(11):1908–1917
Liu C, Li Q, Niu C, Tian Y, Zhao Y, Yin X (2018) The use of atmospheric and room temperature plasma mutagenesis to create a brewing yeast with reduced acetaldehyde production. J Inst Brew 124(3):236–243
Liu P, Wen J, Chen Y, Jia X (2013) Femtosecond laser-based mutagenesis strategy for micronomicin production enhancement of Micromonospora sagamiensis ATCC 21826. World JMicrobiol Biot 29(6):1121–1127
Liu RS, Jin GH, Xiao DR, Li HM, Bai FW, Tang YJ (2015) Screening of the key volatile organic compounds of Tuber melanosporum fermentation by aroma sensory evaluation combination with principle component analysis. Sci Rep 5:17954
Liu SD, Wu YN, Wang TM, Zhang C, Xing XH (2017a) Maltose utilization as a novel selection strategy for continuous evolution of microbes with enhanced metabolite production. ACS Synth Biol 6(12):2326–2338
Liu X, Lv J, Xu J, Xia J, Dai B, Xu X, Xu J (2017b) Erythritol production by Yarrowia lipolytica mutant strain M53 generated through atmospheric and room temperature plasma mutagenesis. Food Sci Biot 26(4):979–986
Liu ZQ, Zhang JF, Zheng YG, Shen YC (2008) Improvement of astaxanthin production by a newly isolated Phaffia rhodozyma mutant with low-energy ion beam implantation. J Appl Microbiol 104(3):861–872
Long Q, Liu X, Yang Y, Li L, Harvey L, McNeil B, Bai Z (2014) The development and application of high throughput cultivation technology in bioprocess development. J Biotechnol 192(Pt B):323–338
Lv X, Song J, Yu B, Liu H, Li C, Zhuang Y, Wang Y (2016) High-throughput system for screening of high L-lactic acid-productivity strains in deep-well microtiter plates. Bioproc Biosyst Eng 39(11):1737–1747
Mays ZJ, Nair NU (2018) Synthetic biology in probiotic lactic acid bacteria: at the frontier of living therapeutics. Curr Opin Biotech 53:224–231
Mertens R, Liese A (2004) Biotechnological applications of hydrogenases. Curr Opin Biotech 15(4):343–348
Mitsui R, Yamada R, Ogino H (2019) CRISPR system in the yeast Saccharomyces cerevisiae and its application in the bioproduction of useful chemicals. World J Microbiol Biotechnol 35(7):111
Murata H, Abe T, Ichida H, Hayashi Y, Yamanaka T, Shimokawa T, Tahara K (2018) Heavy-ion beam mutagenesis of the ectomycorrhizal agaricomycete Tricholoma matsutake that produces the prized mushroom “matsutake” in conifer forests. Mycorrhiza 28(2):171–177
Mustafi N, Grunberger A, Kohlheyer D, Bott M, Frunzke J (2012) The development and application of a single-cell biosensor for the detection of L-methionine and branched-chain amino acids. Metab Eng 14(4):449–457
Neelam, Chhillar AK, Rana JS (2019) Enzyme nanoparticles and their biosensing applications: a review. Anal Biochem 581:113345
Ohnuki S, Kashima M, Yamada T, Ghanegolmohammadi F, Zhou Y, Goshima T, Maruyama J, Kitamoto K, Hirata D, Akao T, Ohya Y (2019) Genome editing to generate nonfoam-forming sake yeast strains. Biosci Biotechnol Biochem 83(8):1583–1593
Pandey KR, Naik SR, Vakil BV (2015) Probiotics, prebiotics and synbiotics—a review. J Food Sci Tech 52(12):7577–7587
Puskeiler R, Kaufmann K, Weuster-Botz D (2005) Development, parallelization, and automation of a gas-inducing milliliter-scale bioreactor for high-throughput bioprocess design (HTBD). Biotechnol Bioeng 89(5):512–523
Qian W, Fu Y, Cai C (2013) Engineering a high-yield glutathione strain of Hansenula polymorpha using ion beam implantation. Prep Biochem Biotechnol 43(6):577–585
Qiang W, Ling-ran F, Luo W, Han-guang L, Lin W, Ya Z, Xiao-bin Y (2014) Mutation breeding of lycopene-producing strain Blakeslea trispora by a novel atmospheric and room temperature plasma (ARTP). Appl Biochem Biotech 174(1):452–460
Qiao Y, Zhao X, Zhu J, Tu R, Dong L, Wang L, Dong Z, Wang Q, Du W (2017) Fluorescence-activated droplet sorting of lipolytic microorganisms using a compact optical system. Lab Chip 18(1):190–196
Rühmann B, Schmid J, Sieber V (2015) Methods to identify the unexplored diversity of microbial exopolysaccharides. Front Microbiol 6:565
Rohe P, Venkanna D, Kleine B, Freudl R, Oldiges M (2012) An automated workflow for enhancing microbial bioprocess optimization on a novel microbioreactor platform. MicrobCell Fact 11:14
Schäpper D, Alam MNHZ, Szita N, Eliasson Lantz A, Gernaey KV (2009) Application of microbioreactors in fermentation process development: a review. Anal Bioanal Chem 395(3):679–695
Schallmey M, Frunzke J, Eggeling L, Marienhagen J (2014) Looking for the pick of the bunch: high-throughput screening of producing microorganisms with biosensors. Curr Opin Biotech 26:148–154
Schleputz T, Buchs J (2014) Scale-down of vinegar production into microtiter plates using a custom-made lid. J Biosci Bioeng 117(4):485–496
Shi F, Tan J, Chu J, Wang Y, Zhuang Y, Zhang S (2015) A qualitative and quantitative high-throughput assay for screening of gluconate high-yield strains by Aspergillus niger. J Microbiol Meth 109:134–139
Shibasaki S, Ueda M (2014) Bioadsorption strategies with yeast molecular display technology. Biocontrol Sci 19:157–164
Siedler S, Khatri NK, Zsohar A, Kjaerbolling I, Vogt M, Hammar P, Nielsen CF, Marienhagen J, Sommer MOA, Joensson HN (2017) Development of a bacterial biosensor for rapid screening of yeast p-coumaric acid production. ACS Synth Biol 6(10):1860–1869
Sjostrom SL, Bai Y, Huang M, Liu Z, Nielsen J, Joensson HN, Andersson Svahn H (2014) High-throughput screening for industrial enzyme production hosts by droplet microfluidics. Lab Chip 14(4):806–813
Song T-T, Di W, He-Nan Z, Wen-Han W, Yan-Fang L, Qiao-Zhen L, Yan Y (2018) Physicochemical properties and immunological activities in vitro of mycelial polysaccharides from Hericium erinaceus mutants induced by atmospheric and room temperature plasma. Mycosystema 37(6):794–804
Spadiut O, Nguyen T, Haltrich D (2010) Thermostable variants of pyranose 2-oxidase showing altered substrate selectivity for glucose and galactose. J Agric Food Chem 58:3465–3471
Strianese M, Zauner G, Tepper AW, Bubacco L, Breukink E, Aartsma TJ, Canters GW, Tabares LC (2009) A protein-based oxygen biosensor for high-throughput monitoring of cell growth and cell viability. Anal Biochem 385(2):242–248
Su C, Zhou W, Fan Y, Wang L, Zhao S, Yu Z (2006) Mutation breeding of chitosanase-producing strain Bacillus sp. S65 by low-energy ion implantation. J Ind Microbiol Biotech 33(12):1037–1042
Sun J, Wang Y, Wu B, Bai Z, He B (2015) Enhanced production of D-lactic acid by Sporolactobacillus sp.Y2–8 mutant generated by atmospheric and room temperature plasma. Biotech Appl Biochem 62(2):287–292
Tan Y, Fang M, Jin L, Zhang C, Li H-P, Xing X-H (2015) Culture characteristics of the atmospheric and room temperature plasma-mutated Spirulina platensis mutants in CO2 aeration culture system for biomass production. J Biosci Bioeng 120(4):438–443
Trček J, Lipoglavšek L, Avguštin G (2016) 16S rRNA in situhybridization followed by flow cytometry for rapid identification of acetic acid bacteria involved in submerged industrial vinegar production. Food Technol Biotech 54(1):108–112
Varela J, Varela C (2019) Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr Opin Biotech 56:88–96
von Wright A, Bruce Å (2003) 7. Genetically modified microorganisms and their potential effects on human health and nutrition. Trends Food Sci Tech 14(5–8):264–276
Wang J, Li R, Lu D, Ma S, Yan Y, Li W (2009a) A quick isolation method for mutants with high lipid yield in oleaginous yeast. World J Microbiol Biotech 25(5):921–925
Wang L, Chen X, Wu G, Li S, Zeng X, Ren X, Tang L, Mao Z (2015) Improved ε-poly-l-lysine production of Streptomyces sp. FEEL-1 by atmospheric and room temperature plasma mutagenesis and streptomycin resistance screening. Ann Microbiol 65(4):2009–2017
Wang P, Li J, Wang L, Tang ML, Yu ZL, Zheng ZM (2009b) L(+)-lactic acid production by co-fermentation of glucose and xylose with Rhizopus oryzae obtained by low-energy ion beam irradiation. J Ind Microbiol Biot 36(11):1363–1368
Wang S, Luo Q, Liu J, Liu L, Chen X (2018) Mutation and fermentation optimization of Bacillus amyloliquefaciens for acetoin production. Chinese J Biot 34(5):803–811
Wang X, Lu M, Wang S, Fang Y, Wang D, Ren W, Zhao G (2014) The atmospheric and room-temperature plasma (ARTP) method on the dextranase activity and structure. Int J Biol Macromol 70:284–291
Wang Z, Zhang L, Tan T (2010) Efficient screening a high glutathione-content mutant of Saccharomyces cerevisiae by flow cytometry. Process Biochem 45(7):1168–1171
Wittmann C, Kim HM, Heinzle E (2004) Metabolic network analysis of lysine producing Corynebacterium glutamicum at a miniaturized scale. Biotechnol Bioeng 87(1):1–6
Wu B, Qin H, Yang Y, Duan G, Yang S, Xin F, Zhao C, Shao H, Wang Y, Zhu Q, Tan F, Hu G, He M (2019) Engineered Zymomonas mobilis tolerant to acetic acid and low pH via multiplex atmospheric and room temperature plasma mutagenesis. Biotechnol Biofuels 12(1)
Wu X, Wei Y, Xu Z, Liu L, Tan Z, Jia S (2015) Evaluation of an ethanol-tolerant Acetobacter pasteurianus mutant generated by a new atmospheric and room temperature plasma (ARTP). Adv Appl Biot 333:277–286
Xu J-Z, Zhang W-G (2017) Menaquinone-7 production from maize meal hydrolysate by Bacillus isolates with diphenylamine and analogue resistance. J Zhejiang Univ-Sci B 18(6):462–473
Yu G, Jia X, Wen J, Lu W, Wang G, Caiyin Q, Chen Y (2010) Strain improvement of Streptomyces roseosporus for daptomycin production by rational screening of He-Ne laser and NTG induced mutants and kinetic modeling. Appl Biochem Biotech 163(6):729–743
Zeng W, Du G, Chen J, Li J, Zhou J (2015) A high-throughput screening procedure for enhancing α-ketoglutaric acid production in Yarrowia lipolytica by random mutagenesis. Process Biochem 50(10):1516–1522
Zhang C, Shen H, Zhang X, Yu X, Wang H, Xiao S, Wang J, Zhao ZK (2016) Combined mutagenesis of Rhodosporidium toruloides for improved production of carotenoids and lipids. Biotechnol Lett 38(10):1733–1738
Zhang G, Lin Y, Qi X, Wang L, He P, Wang Q, Ma Y (2015a) Genome shuffling of the nonconventional yeast Pichia anomala for improved sugar alcohol production. Microb Cell Fact 14(1):112
Zhang J, Jensen MK, Keasling JD (2015b) Development of biosensors and their application in metabolic engineering. Curr Opin Chem Biol 28:1–8
Zhang M, Li Q, Zhou Z, Lu L, Du G, Chen J, Fang F (2017) Reduction of ethyl carbamate in soy sauce by Bacillus amyloliquefaciens mutants. Acta Microbiol Sin 57(12):1817–1826
Zhang M, Xiao Y, Zhu R, Zhang Q, Wang SL (2012a) Enhanced thermotolerance and ethanol tolerance in Saccharomyces cerevisiae mutated by high-energy pulse electron beam and protoplast fusion. Bioprocess Biosyst Eng 35(9):1455–1465
Zhang M, Zhu R, Zhang M, Gao B, Sun D, Wang S (2013) High-energy pulse-electron-beam-induced molecular and cellular damage in Saccharomyces cerevisiae. Res Microbiol 164(2):100–109
Zhang N, Jiang J-C, Yang J, Wei M, Zhao J, Xu H, Xie J-C, Tong Y-J, Yu L (2018a) Citric acid production from acorn starch by tannin tolerance mutant Aspergillus niger AA120. Appl Biochem Biotechnol 188(1):1–11
Zhang Q, Fu Y, Wang Y, Han J, Lv J, Wang S (2012b) Improved ethanol production of a newly isolated thermotolerant Saccharomyces cerevisiae strain after high-energy-pulse-electron beam. J Appl Microbiol 112(2):280–288
Zhang X, Zhang C, Zhou QQ, Zhang XF, Wang LY, Chang HB, Li HP, Oda Y, Xing XH (2015c) Quantitative evaluation of DNA damage and mutation rate by atmospheric and room-temperature plasma (ARTP) and conventional mutagenesis. Appl Microbiol Biot 99(13):5639–5646
Zhang X, Zhang X, Xu G, Zhang X, Shi J, Xu Z (2018b) Integration of ARTP mutagenesis with biosensor-mediated high-throughput screening to improve L-serine yield in Corynebacterium glutamicum. Appl Microbiol Biot 102(14):5939–5951
Zhang X, Zhang XF, Li HP, Wang LY, Zhang C, Xing XH, Bao CY (2014) Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl Microbiol Biot 98(12):5387–5396
Zhao B, Li Y, Li C, Yang H, Wang W (2018) Enhancement of Schizochytrium DHA synthesis by plasma mutagenesis aided with malonic acid and zeocin screening. Appl Microbiol Biot 102(5):2351–2361
Zhao G, Hou L, Lu M, Wei Y, Zeng B, Wang C, Cao X (2012) Construction of the mutant strain in Aspergillus oryzae 3.042 for abundant proteinase production by the N+ ion implantation mutagenesis. Int J Food Sci Tech 47(3):504–510
Zhu X, Arman B, Chu J, Wang Y, Zhuang Y (2017) Development of a method for efficient cost-effective screening of Aspergillus niger mutants having increased production of glucoamylase. Biotechnol Lett 39(5):739–744
Zhou D, Jiang Z, Pang Q, Zhu Y, Wang Q, Qi Q (2019) CRISPR/Cas9-assisted seamless genome editing in Lactobacillus plantarum and its application in N-acetylglucosamine production. Appl Environ Microb 85(21):e01367–e01319
Funding
This work is supported by the National Natural Science Foundation of China (31801532), Sichuan Provincial Science and Technology Project (2017NZ0036), Agricultural Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2018-BIOMA), and Open Funds of the Key Laboratory of Development and Application of Rural Renewable Energy of Agriculture, Ministry of Agriculture, China (2017002&2018-009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Q., Li, Y., Wu, B. et al. Novel mutagenesis and screening technologies for food microorganisms: advances and prospects. Appl Microbiol Biotechnol 104, 1517–1531 (2020). https://doi.org/10.1007/s00253-019-10341-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10341-z