Abstract
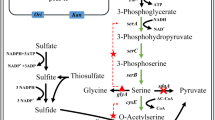
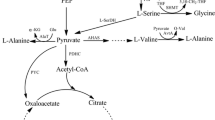
l-Cysteine is a commercially important amino acid. Here, we report the construction of l-cysteine-producing Corynebacterium glutamicum using a metabolic engineering approach. l-Serine O-acetyltransferase (SAT), encoded by cysE gene, is a key enzyme of l-cysteine biosynthesis, because of its feedback inhibition by l-cysteine. Therefore, we introduced a mutation into the C. glutamicum cysE gene, which appeared to desensitize SAT against feedback inhibition by l-cysteine. We successfully produced l-cysteine by overexpressing this mutant cysE gene in C. glutamicum, while the wild-type strain scarcely produced l-cysteine. To enhance the biosynthesis of l-serine (a substrate for SAT), a mutant serA gene, encoding D-3-phosphoglycerate dehydrogenase to desensitize it against feedback inhibition by l-serine, was additionally overexpressed in the mutant cysE-overexpressing strain and its l-cysteine production was indeed improved. Moreover, we disrupted the ldh gene encoding l-lactate dehydrogenase and the aecD gene encoding cysteine desulfhydrase to prevent the formation of lactic acid as a by-product and degradation of l-cysteine produced at the stationary phase, respectively, which resulted in enhanced l-cysteine production. However, since the concentration of l-cysteine produced still decreased at the stationary phase despite the aecD disruption, NCgl2463 encoding a possible cystine importer protein was further disrupted to prevent cystine import, because the produced l-cysteine is immediately oxidized to cystine. As a result, the time before the start of the decrease in l-cysteine concentration was successfully prolonged. Approximately 200 mg/L of l-cysteine production was achieved by overexpression of mutant cysE and serA genes and disruption of aecD and NCgl2463 genes in C. glutamicum.






Similar content being viewed by others
References
Cheah IK, Halliwell B (2012) Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta 1822:784–793. https://doi.org/10.1016/j.bbadis.2011.09.017
Chonoles Imlay KR, Korshunov S, Imlay JA (2015) Physiological roles and adverse effects of the two cystine importers of Escherichia coli. J Bacteriol 197:3629–3644. https://doi.org/10.1128/jb.00277-15
Eggeling L, Bott M (2005) Handbook of Corynebacterium glutamicum. CRC press
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Genghof DS (1970) Biosynthesis of ergothioneine and hercynine by fungi and Actinomycetales. J Bacteriol 103:475–478
Gorman J, Shapiro L (2004) Structure of serine acetyltransferase from Haemophilus influenzae Rd. Acta Crystallogr D Biol Crystallogr 60:1600–1605. https://doi.org/10.1107/s0907444904015240
Hennicke F, Grumbt M, Lermann U, Ueberschaar N, Palige K, Böttcher B, Jacobsen ID, Staib C, Morschhäuser J, Monod M, Hube B, Hertweck C, Staib P (2013) Factors supporting cysteine tolerance and sulfite production in Candida albicans. Eukaryot Cell 12:604–613
Hunt S (1985) Degradation of amino acids accompanying in vitro protein hydrolysis. In: Barrett GC (ed) Chemistry and biochemistry of the amino acids. Springer, pp 376–398
Joo YC, Hyeon JE, Han SO (2017) Metabolic design of Corynebacterium glutamicum for production of L-cysteine with consideration of sulfur-supplemented animal feed. J Agric Food Chem 65:4698–4707. https://doi.org/10.1021/acs.jafc.7b01061
Kawano Y, Onishi F, Shiroyama M, Miura M, Tanaka N, Oshiro S, Nonaka G, Nakanishi T, Ohtsu I (2017) Improved fermentative L-cysteine overproduction by enhancing a newly identified thiosulfate assimilation pathway in Escherichia coli. Appl Microbiol Biotechnol 101:6879–6889. https://doi.org/10.1007/s00253-017-8420-4
Kinoshita S, Udaka S, Shimono M (1957) Studies on the amino acid fermentation. Part 1. Production of L-glutamic acid by various microorganisms. J Gen Appl Microbiol 50:193–205
Nakamori S, Kobayashi S-i, Kobayashi C, Takagi H (1998) Overproduction of L-cysteine and L-cystine by Escherichia coli strains with a genetically altered serine acetyltransferase. Appl Environ Microbiol 64:1607–1611
Nakayama K, Kitada S, Kinoshita S (1961) Studies on lysine fermentation I. The control mechanism on lysine accumulation by homoserine and threonine. J Gen Appl Microbiol 7:145–154
Ohtsu I, Kawano Y, Suzuki M, Morigasaki S, Saiki K, Yamazaki S, Nonaka G, Takagi H (2015) Uptake of L-cystine via an ABC transporter contributes defense of oxidative stress in the L-cystine export-dependent manner in Escherichia coli. PLoS One 10:e0120619. https://doi.org/10.1371/journal.pone.0120619
Osawa R, Kamide T, Satoh Y, Kawano Y, Ohtsu I, Dairi T (2018) Heterologous and high production of ergothioneine in Escherichia coli. J Agric Food Chem 66:1191–1196. https://doi.org/10.1021/acs.jafc.7b04924
Park S, Imlay JA (2003) High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J Bacteriol 185:1942–1950
Peters-Wendisch P, Netzer R, Eggeling L, Sahm H (2002) 3-Phosphoglycerate dehydrogenase from Corynebacterium glutamicum: the C-terminal domain is not essential for activity but is required for inhibition by L-serine. Appl Microbiol Biotechnol 60:437–441. https://doi.org/10.1007/s00253-002-1161-y
Pluskal T, Ueno M, Yanagida M (2014) Genetic and metabolomic dissection of the ergothioneine and selenoneine biosynthetic pathway in the fission yeast, S. pombe, and construction of an overproduction system. PLoS One 9:e97774. https://doi.org/10.1371/journal.pone.0097774
Pye VE, Tingey AP, Robson RL, Moody PC (2004) The structure and mechanism of serine acetyltransferase from Escherichia coli. J Biol Chem 279:40729–40736. https://doi.org/10.1074/jbc.M403751200
Rückert C, Milse J, Albersmeier A, Koch DJ, Pühler A, Kalinowski J (2008) The dual transcriptional regulator CysR in Corynebacterium glutamicum ATCC 13032 controls a subset of genes of the McbR regulon in response to the availability of sulphide acceptor molecules. BMC Genomics 9:483. https://doi.org/10.1186/1471-2164-9-483
Sato H, Orishimo K, Shirai T, Hirasawa T, Nagahisa K, Shimizu H, Wachi M (2008) Distinct roles of two anaplerotic pathways in glutamate production induced by biotin limitation in Corynebacterium glutamicum. J Biosci Bioeng 106:51–58. https://doi.org/10.1263/jbb.106.51
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Shiio I, Nakamori S (1970) Microbial production of L-threonine. Part II. Production by α-amino-β-hydroxyvaleric acid resistant mutants of glutamate producing bacteria. Agric Biol Chem 34:448–456
Sørensen MA, Pedersen S (1991) Cysteine, even in low concentrations, induces transient amino acid starvation in Escherichia coli. J Bacteriol 173:5244–5246
Sperandio B, Gautier C, Pons N, Ehrlich DS, Renault P, Guedon E (2010) Three paralogous LysR-type transcriptional regulators control sulfur amino acid supply in Streptococcus mutans. J Bacteriol 192:3464–3473. https://doi.org/10.1128/JB.00119-10
Takagi H, Ohtsu I (2017) L-Cysteine metabolism and fermentation in microorganisms. In: Yokota A, Ikeda M (eds) Advances in biochemical engineering/biotechnology. Vol 159. Springer nature, pp 129–151
Takumi K, Ziyatdinov MK, Samsonov V, Nonaka G (2017) Fermentative production of cysteine by Pantoea ananatis. Appl Environ Microbiol 83. https://doi.org/10.1128/aem.02502-16
Tsuchida T, Yoshinaga F, Kubota K, Momose H (1975) Production of L-valine by 2-thiazolealanine resistant mutants derived from glutamic acid producing bacteria. Agric Biol Chem 39:1319–1322
Udaka S (1960) Screening method for microorganisms accumulating metabolites and its use in the isolation of Micrococcus glutamicus. J Bacteriol 79:754–755
Wada M, Awano N, Haisa K, Takagi H, Nakamori S (2002) Purification, characterization and identification of cysteine desulfhydrase of Corynebacterium glutamicum, and its relationship to cysteine production. FEMS Microbiol Lett 217:103–107
Wiriyathanawudhiwong N, Ohtsu I, Li ZD, Mori H, Takagi H (2009) The outer membrane TolC is involved in cysteine tolerance and overproduction in Escherichia coli. Appl Microbiol Biotechnol 81:903–913. https://doi.org/10.1007/s00253-008-1686-9
Acknowledgements
Authors thank Professor Iwao Ohtsu and Dr. Yusuke Kawano (University of Tsukuba) for their technical support in measurement of l-cysteine concentration by Gaitonde method, and the Biomaterials Analysis Division (Tokyo Institute of Technology) for technical assistance in DNA sequence analysis.
Funding
This work was financially supported by Science and Technology Research Promotion Program from the Ministry of Agriculture, Forestry and Fisheries of Japan. It was also supported in part by JSPS KAKENHI Grant Number JP16K14881.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 266 kb)
Rights and permissions
About this article
Cite this article
Kondoh, M., Hirasawa, T. l-Cysteine production by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 103, 2609–2619 (2019). https://doi.org/10.1007/s00253-019-09663-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09663-9